1
answer
0
watching
297
views
6 Oct 2020
The Balmer series for the hydrogen atom corresponds to electronic transitions that terminate in the state with quantum number n = 2 as shown in Figure P41.7 (page 1140). Consider the photon of longest wavelength corresponding to a transition shown in the figure. Determine (a) its energy and (b) its wavelength. Consider the spectral line of shortest wavelength corresponding to a transition shown in the figure. Find (c) its photon energy and (d) its wavelength. (e) What is the shortest possible wavelength in the Balmer series?
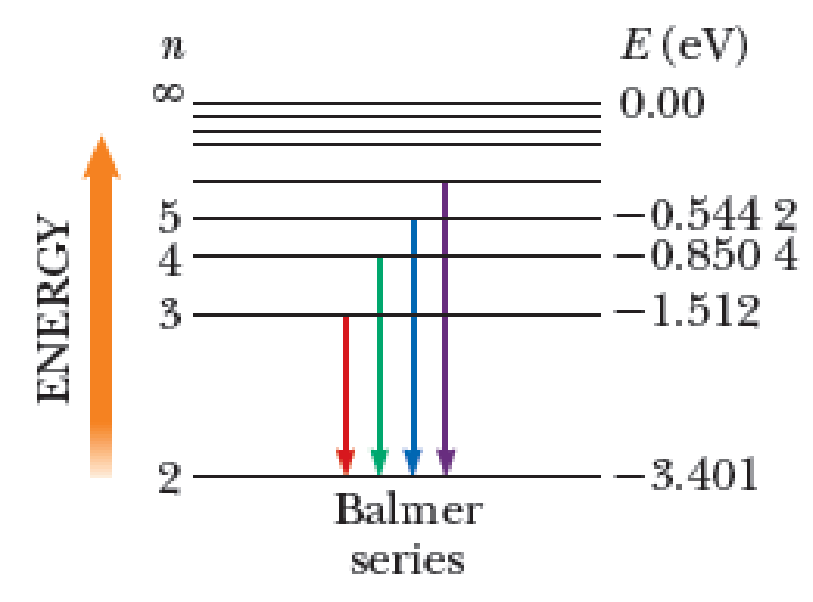
Figure P41.7
The Balmer series for the hydrogen atom corresponds to electronic transitions that terminate in the state with quantum number n = 2 as shown in Figure P41.7 (page 1140). Consider the photon of longest wavelength corresponding to a transition shown in the figure. Determine (a) its energy and (b) its wavelength. Consider the spectral line of shortest wavelength corresponding to a transition shown in the figure. Find (c) its photon energy and (d) its wavelength. (e) What is the shortest possible wavelength in the Balmer series?

Figure P41.7
Ankit LalLv10
7 Mar 2021



