CHEM 101 Midterm: CHEM 101 EXAM 3 CHAPTER 8
Document Summary
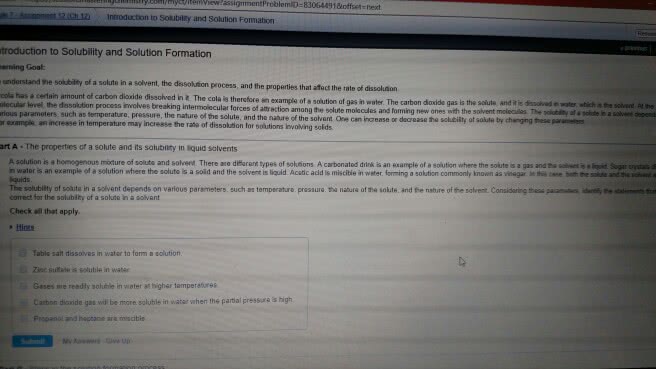
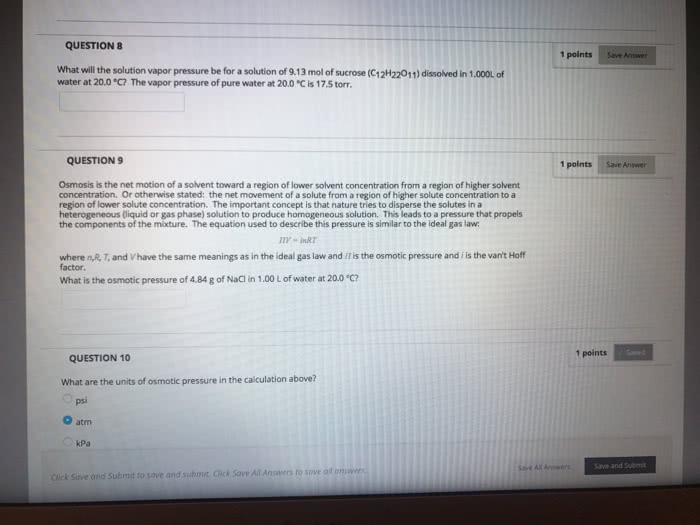
Chem 101 exam 3 review sheet (chapter 8) Intermingling of components at the particle level is a requirement for homogeneity: the solutes remain uniformly distributed throughout the solution and will not settle out with time, the solute(s) generally can be separated from the solvent by physical. Compounds containing the following ions are soluble with exceptions as noted. Compounds containing the following ions are insoluble with exceptions as noted. 5. 0ml of alcohol dissolved in enough water to give 100ml of solution: mass-volume percent, %(m/v) = [mass of solute(g)/volume of solution(ml)] x 100, a 2. 3%(m/v) solution of any solute contains 2. 3g of solute in each. 100ml of solution: conversion factors obtained from percent. 25g sucrose/100ml solution and 100ml solution/25g sucrose: molarity: the moles of the solute in a solution divided by the liters of solution. There are 6ml of methanol in 100ml of solution. There are 25g of sucrose in 100ml of solution.