Videos
Expert Answer
General guidance
The concept used to solve this problem is determined the Conjugated acid-Base.
Bronsted-Lowry acid-base system:
Acid:
According to Bronsted-Lowry; acid is a substance that donates the 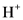 (hydrogen ion) or proton. In general Bronsted-Lowry acid is a proton donor.
(hydrogen ion) or proton. In general Bronsted-Lowry acid is a proton donor.
Base:
According to Bronsted-Lowry; base is a substance that accepts the 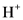 (hydrogen ion) or proton. In general Bronsted-Lowry base is a proton acceptor.
(hydrogen ion) or proton. In general Bronsted-Lowry base is a proton acceptor.
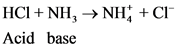
Conjugated acid-Base Pairs:
The pair of Acid-Base which is differs by a proton is called conjugated Acid-Base pair. The acid of conjugated acid-base pair has one more hydrogen atom from base of conjugated acid-base pair.
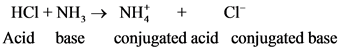
After donate one proton from an acid conjugate base will form; thus, 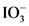 is the conjugate base of
is the conjugate base of 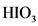 .
.
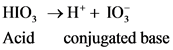
After accepts one proton by a base conjugate acid will form; thus 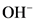 is the conjugate acid of
is the conjugate acid of 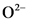 .
.
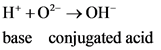
Step-by-step
Step 1 of 4
Part a
The conjugate acid of 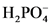 will from after accept one proton by
will from after accept one proton by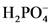 as follows:
as follows:
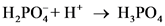
After donate one proton from an acid conjugate base will form and after accepts one proton by a base conjugate acid will form.
The pair of Acid-Base which is differs by a proton is called conjugated Acid-Base pair. The acid of conjugated acid-base pair has one more hydrogen atom from base of conjugated acid-base pair.
Step 2 of 4
The conjugate acid of 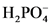 will from after accept one proton by
will from after accept one proton by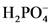 as follows:
as follows:
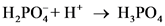
Base Conjugate acid
The conjugate acid of 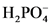 is
is 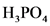 .
.
Explanation
The pair of Acid-Base which is differs by a proton is called conjugated Acid-Base pair. The acid of conjugated acid-base pair has one more hydrogen atom from base of conjugated acid-base pair.
Step 3 of 4
Part b
The conjugate base of 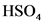 will from after donate one proton from
will from after donate one proton from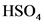 as follows:
as follows:
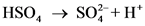
The pair of Acid-Base which is differs by a proton is called conjugated Acid-Base pair. The acid of conjugated acid-base pair has one more hydrogen atom from base of conjugated acid-base pair.
Step 4 of 4
The conjugate base of 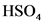 will from after donate one proton from
will from after donate one proton from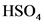 as follows:
as follows:
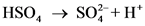
Acid Conjugate base
The conjugate base of 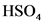 is
is 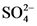 .
.
Explanation | Common mistakes
The pair of Acid-Base which is differs by a proton is called conjugated Acid-Base pair. The acid of conjugated acid-base pair has one more hydrogen atom from base of conjugated acid-base pair.
Answer
The conjugate acid of 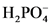 is
is 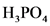 .
.
The conjugate base of 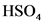 is
is 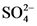 .
.