CHEM 121 Lecture Notes - Lecture 11: Principal Quantum Number, Magnetic Quantum Number, Exponential Decay
CHEM 121 verified notes
11/38View all
Document Summary
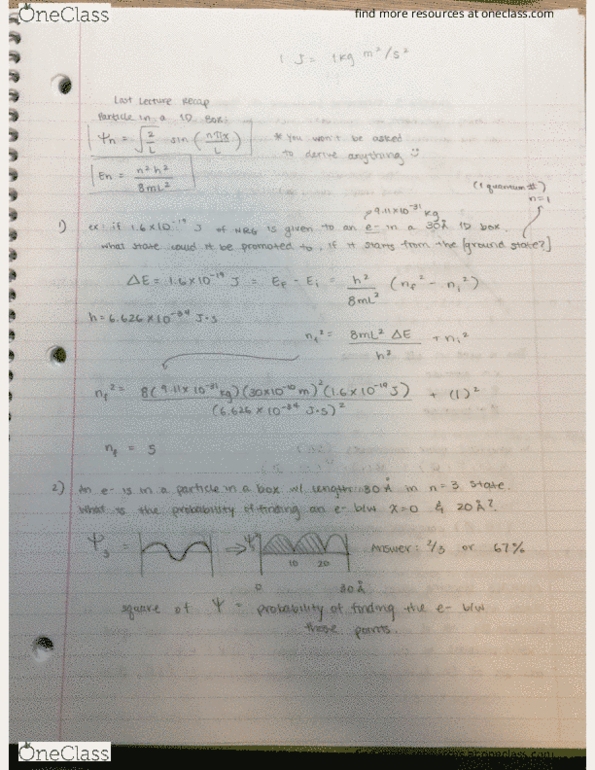
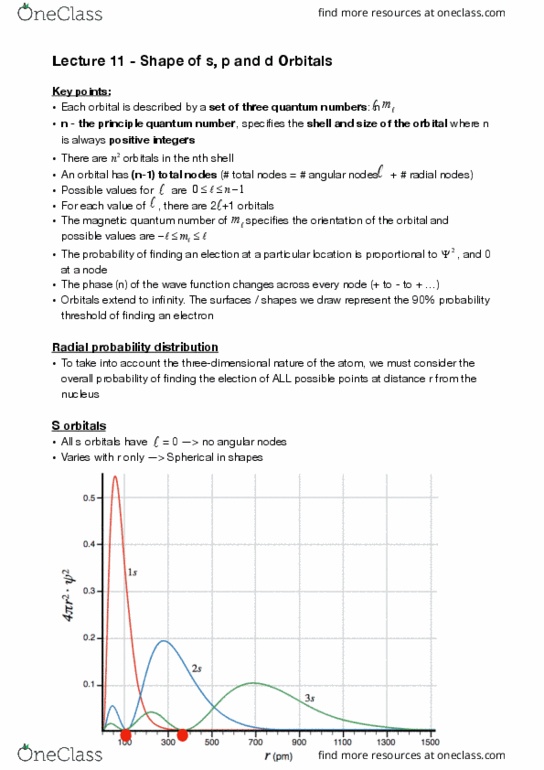
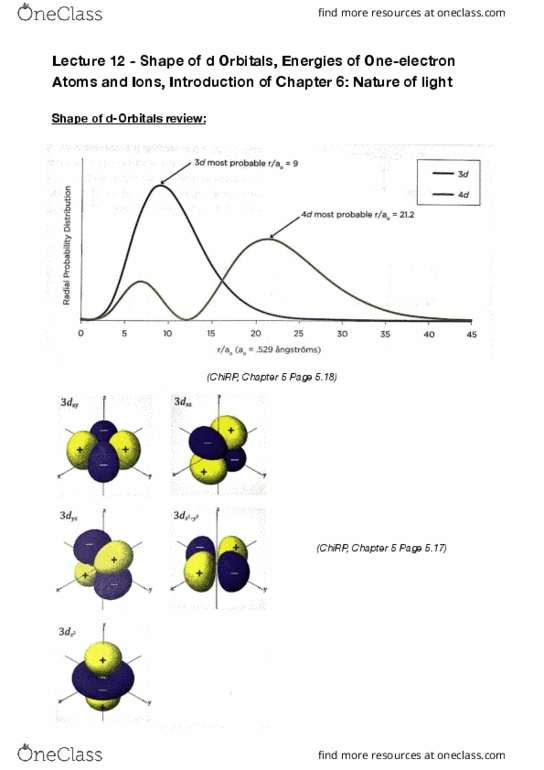
Lecture 11 - shape of s, p and d orbitals. Key points: each orbital is described by a set of three quantum numbers: n. M: n - the principle quantum number, speci es the shell and size of the orbital where n is always positive integers, there are # 2 n orbitals in the nth shell: an orbital has (n-1) total nodes (# total nodes = # angular nodes + # radial nodes, possible values for are. 0 n 1: for each value of , there are 2 +1 orbitals, the magnetic quantum number of speci es the orientation of the orbital and m possible values are. M: the probability of nding an election at a particular location is proportional to # , and 0 at a node: the phase (n) of the wave function changes across every node (+ to - to + , orbitals extend to in nity.