CHEM1006 Lecture Notes - Lecture 20: Isobutane, Chemical Formula, Butane
Document Summary
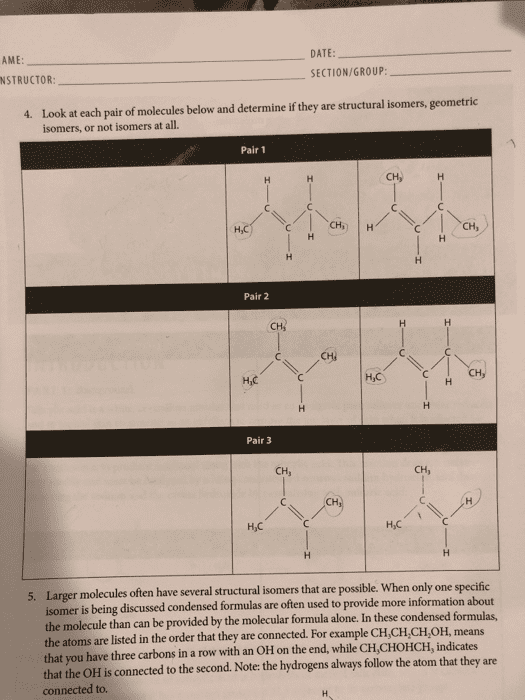
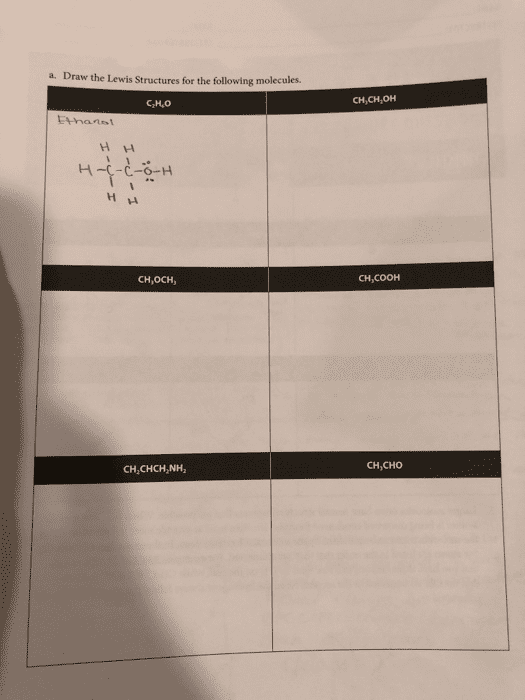
Chemists use a variety of notations to describe and summarize the atomic constituents of compounds. These notations, which include empirical, molecular, and structural formulas, use the chemical symbols for the elements along with numeric values to describe atomic composition. Empirical formulas are the simplest form of notation. They provide the lowest whole-number ratio between the elements in a compound. Unlike molecular formulas, they do not provide information about the absolute number of atoms in a single molecule of a compound. The molecular formula for a compound is equal to, or a whole-number multiple of, its empirical formula. An empirical formula lacks any structural information about the positioning or bonding of atoms in a molecule. It can therefore describe a number of different structures, or isomers, with varying physical properties. For butane and isobutane, the empirical formula for both molecules is c2h5, and they share the same molecular formula, c4h10. Ch3ch2ch2ch3, while isobutane can be described using the structural formula3ch.