CHEM 101 Lecture Notes - Lecture 6: Max Born
CHEM 101 verified notes
6/40View all
Document Summary
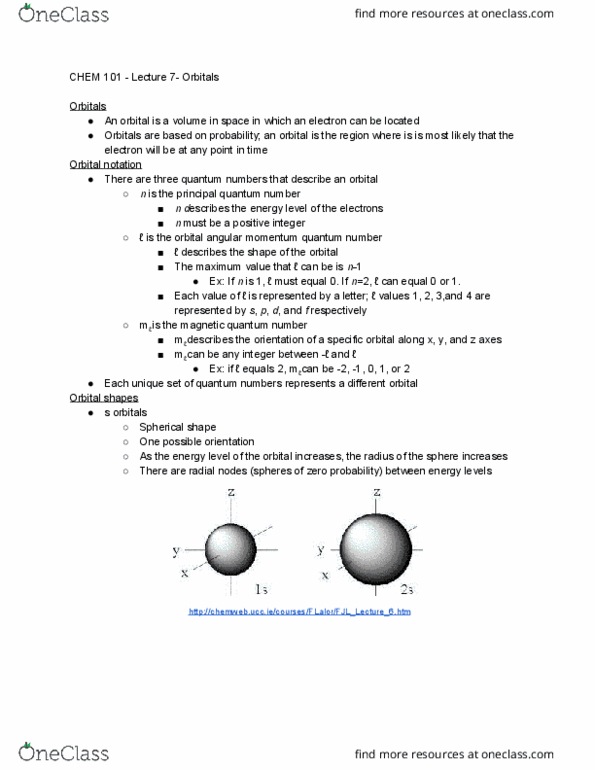
In the early 20th century, many new scientific discoveries were made that advanced quantum mechanics and changed people"s understanding of the atom. This affected how people understood the atomic structure as well as reactions between atoms or molecules. A quantum mechanical description of an electron. Only used for electrons in the hydrogen atom. Treats electrons as standing waves orbiting the nucleus of an atom. The number of crests and troughs of the wave is equal to the the energy level (n) of the electron; as the frequency of the wave increases, the energy level increases. The exact meaning of was not understood. Max born proposed that the wave function was related to the probability of finding an electron in specific positions in an atom. He proposed that 2 is equal to the probability density, or electron density, in the atom.