CHEM 101 Lecture Notes - Lecture 23: Trigonal Planar Molecular Geometry, Orbital Hybridisation, Sigma Bond
CHEM 101 verified notes
23/40View all
22

CHEM 101 Lecture Notes - Lecture 22: Pauli Exclusion Principle, Valence Electron, Vsepr Theory
23
CHEM 101 Lecture Notes - Lecture 23: Trigonal Planar Molecular Geometry, Orbital Hybridisation, Sigma Bond
24
CHEM 101 Lecture Notes - Lecture 24: Bond-Dissociation Energy, Trigonal Bipyramidal Molecular Geometry, Endothermic Process
Document Summary
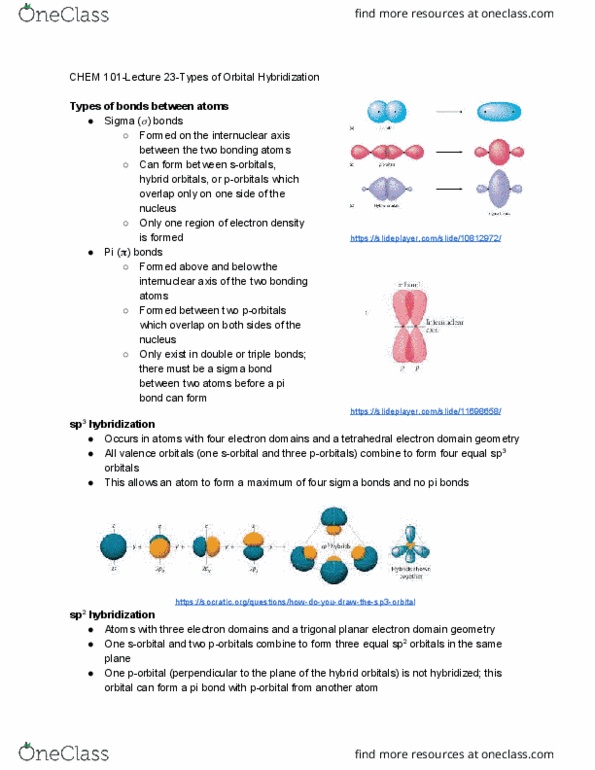
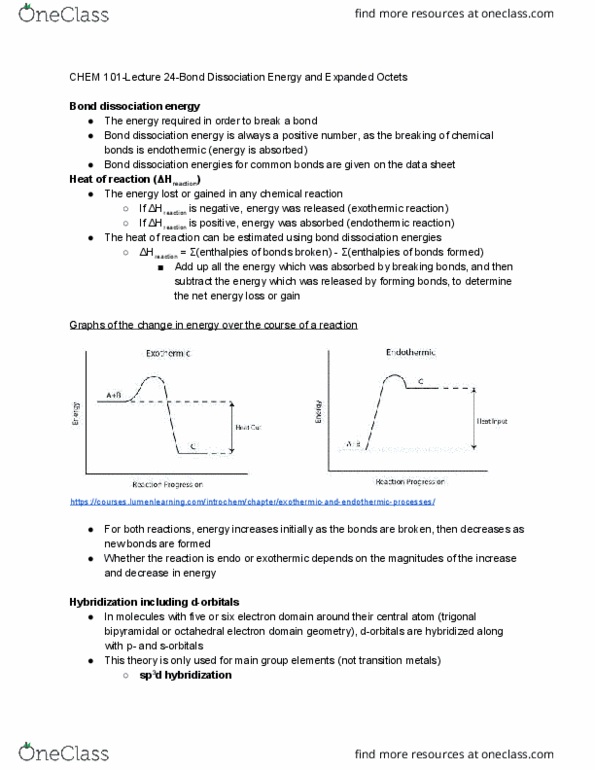
Formed on the internuclear axis between the two bonding atoms. Can form between s-orbitals, hybrid orbitals, or p-orbitals which overlap only on one side of the nucleus. Only one region of electron density is formed. Formed above and below the internuclear axis of the two bonding atoms. Formed between two p-orbitals which overlap on both sides of the nucleus. Only exist in double or triple bonds; there must be a sigma bond between two atoms before a pi bond can form sp . Occurs in atoms with four electron domains and a tetrahedral electron domain geometry. All valence orbitals (one s-orbital and three p-orbitals) combine to form four equal sp 3. This allows an atom to form a maximum of four sigma bonds and no pi bonds orbitals https://slideplayer. com/slide/11698658/ https://socratic. org/questions/how-do-you-draw-the-sp3-orbital. Atoms with three electron domains and a trigonal planar electron domain geometry. One s-orbital and two p-orbitals combine to form three equal sp 2 orbitals in the same.



