CHE 106 Lecture Notes - Lecture 19: Photon, Louis De Broglie, Niels Bohr
CHE 106 verified notes
19/42View all
Document Summary
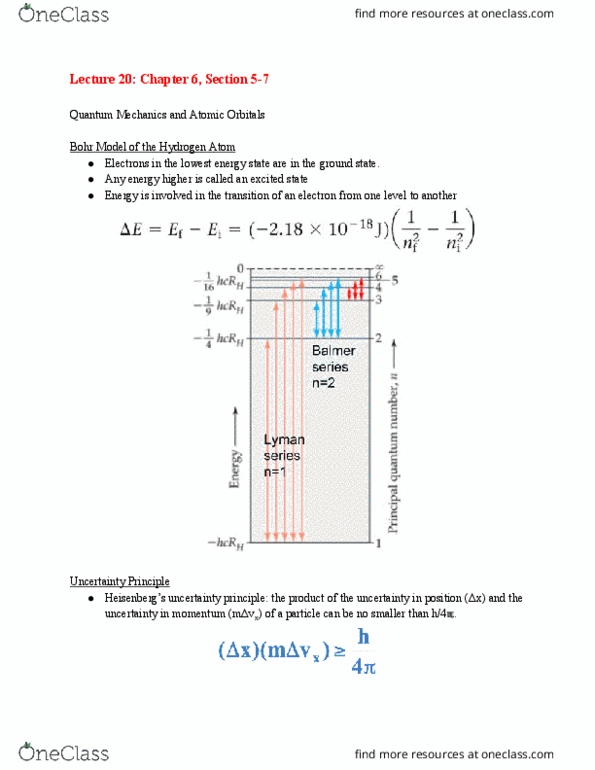
Lecture 19: chapter 6, sections 3 & 4. Light spectra, bohr model, wave behavior of matter. The majority of the mass of the atom was concentrated in a very small nucleus with the straight through the foil, but some were deflected at large angles electrons around the outside. Prior to the work of niels bohr (1922 nobel prize in physics), the stability of the atom could not be explained using the then-current theories. In 1913, using the work of einstein and planck, he applied a new theory to the simplest atom, hydrogen. Experiment support of bohr"s theory: the line spectra of atoms. Bohr derived the following formula for the energy levels of the electron in the hydrogen atom. N = 1, 2, 3 (infinity) for the h atom. R h is the rydberg constant (expressed in energy units) with a value of 2. 18 x 10 -18. Energy-level diagram for the electron in the hydrogen atom.