ENGR 1A Lecture Notes - Lecture 4: Noble Gas, Pauli Exclusion Principle, Atomic Orbital
ENGR 1A verified notes
4/31View all
Document Summary
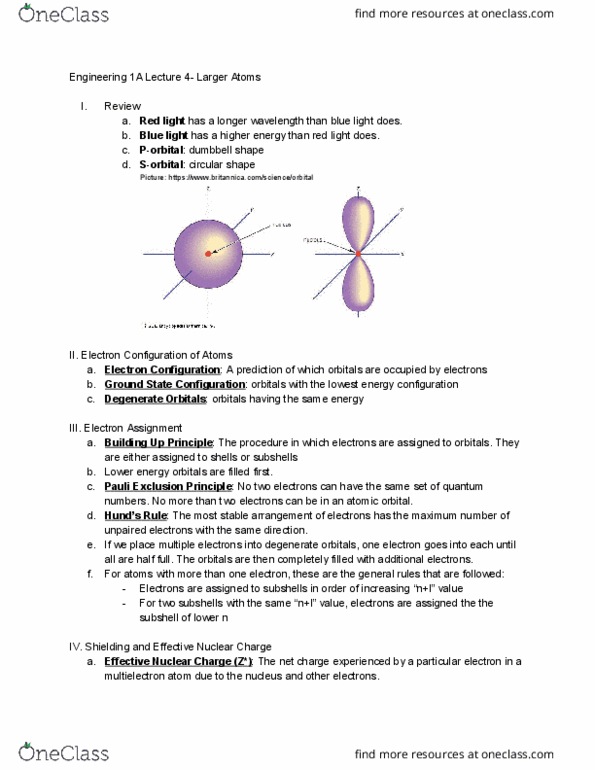
Review: red light has a longer wavelength than blue light does, blue light has a higher energy than red light does, p-orbital : dumbbell shape, s-orbital : circular shape. Electron configuration of atoms: electron configuration : a prediction of which orbitals are occupied by electrons, ground state configuration : orbitals with the lowest energy configuration, degenerate orbitals : orbitals having the same energy. Electron assignment are either assigned to shells or subshells: building up principle : the procedure in which electrons are assigned to orbitals. If we place multiple electrons into degenerate orbitals, one electron goes into each until e. all are half full. The orbitals are then completely filled with additional electrons. numbers. No more than two electrons can be in an atomic orbital: for atoms with more than one electron, these are the general rules that are followed: Electrons are assigned to subshells in order of increasing n+l value.