ENGR 1A Lecture Notes - Lecture 24: Intermolecular Force, Hydrogen Bond, Boiling Point
ENGR 1A verified notes
24/31View all
Document Summary
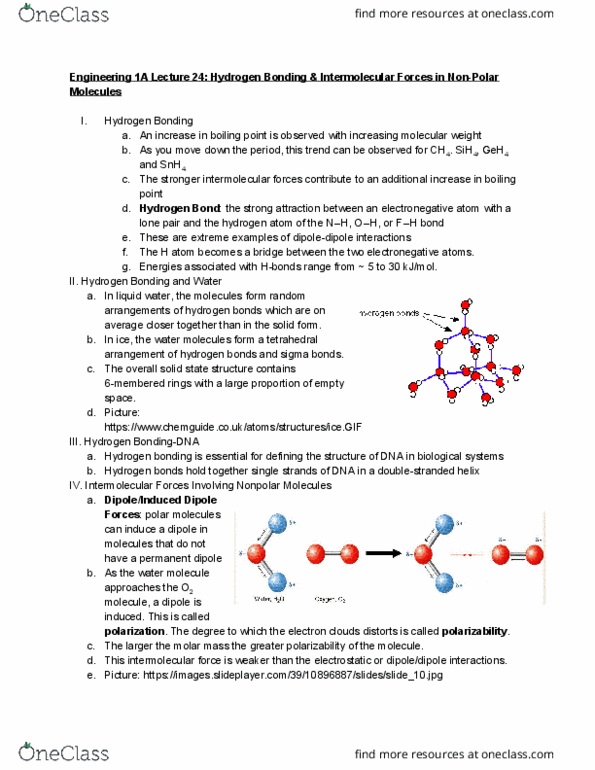
Engineering 1a lecture 24: hydrogen bonding & intermolecular forces in non-polar. Hydrogen bonding: an increase in boiling point is observed with increasing molecular weight, as you move down the period, this trend can be observed for ch 4 . Sih 4 , geh 4: the stronger intermolecular forces contribute to an additional increase in boiling and snh 4. point. In liquid water, the molecules form random arrangements of hydrogen bonds which are on average closer together than in the solid form. In ice, the water molecules form a tetrahedral arrangement of hydrogen bonds and sigma bonds: the overall solid state structure contains. 6-membered rings with a large proportion of empty space: picture: https://www. chemguide. co. uk/atoms/structures/ice. gif. Hydrogen bonding-dna: hydrogen bonding is essential for defining the structure of dna in biological systems, hydrogen bonds hold together single strands of dna in a double-stranded helix. Intermolecular forces involving nonpolar molecules: dipole/induced dipole.