CHEM 1127Q Lecture Notes - Lecture 16: Gravimetric Analysis, Chemical Energy, Sodium Chloride
CHEM 1127Q verified notes
16/27View all
Document Summary
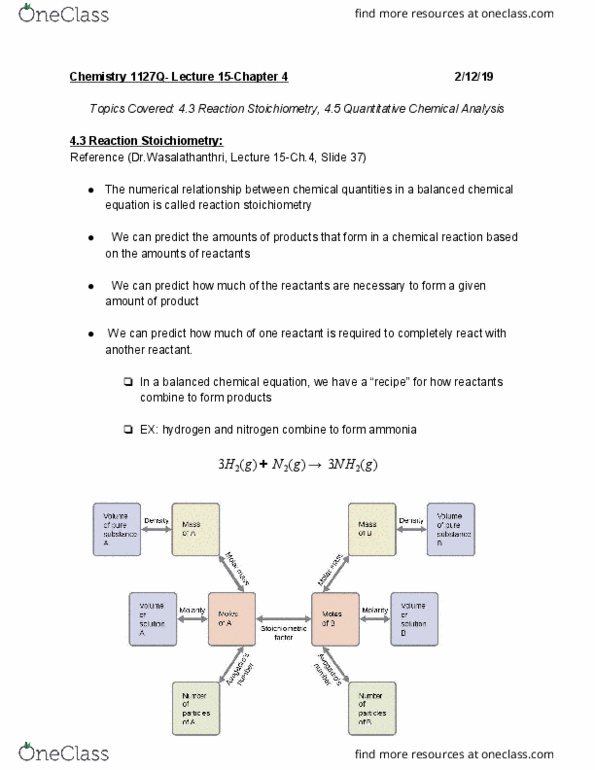
Topics covered: 4. 5 quantitative chemical analysis, 5. 1 energy basics. Gravimetric analysis is one in which a sample is subjected to some treatment that causes a change in the physical state of the analyte that permits its separation from the other components of the sample. The required change of state in a gravimetric analysis may be achieved by various physical and chemical processes. The precipitate is typically isolated from the reaction mixture by filtration, carefully dried, and then weighed. The mass of the precipitate may then be used, along with relevant stoichiometric relationships, to calculate analyte concentration. 3 2 in the original mixture. is dissolved in water. to form 21. 75 g of. No , k no , bacl , baso. A 20. 00 g sample consisting of a mixture of and. This aqueous mixture then reacts with excess aqueous solid. Determine if the products are soluble or insoluble: **if soluble the product will be aqueous, ex. solid**