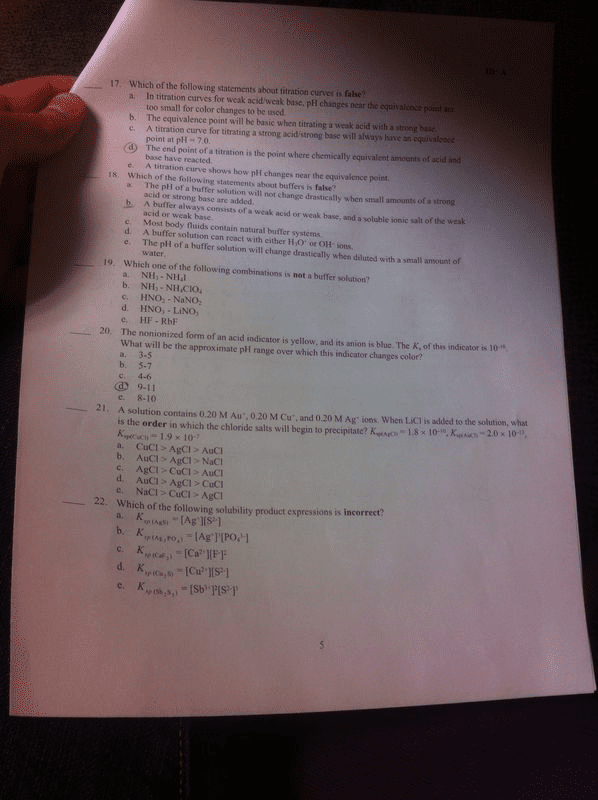CHEM 103 Lecture Notes - Lecture 13: Sodium Acetate, Titration Curve, Buffer Solution
Document Summary
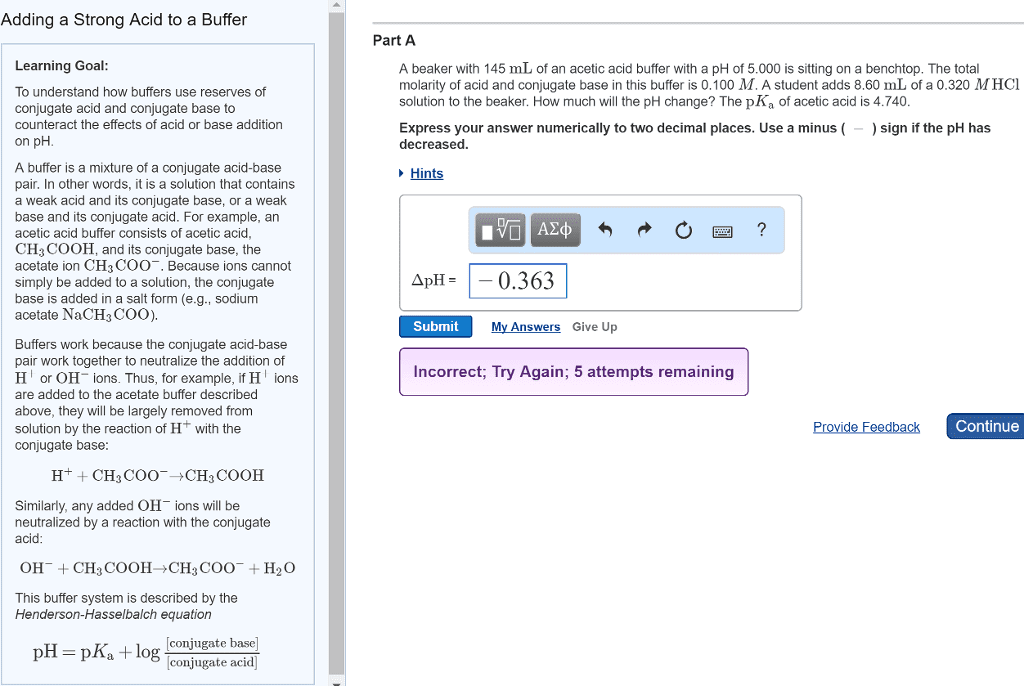
Unit 13 aqueous ionic equilibrium (chapter 16 in tro textbook) Solutions that resist changes in ph when either oh- or h+ ions are added. Usually contain a weak acid and its salt or a weak base and its salt. Addition of strong acid: h+ + nh3 nh4. Pure water has no buffering capacity---acids and bases added to water directly affect the ph of the solution. Buffer capacity- the amount of acid or base that can be absorbed by a buffer system without a significant change in ph. In order to have a large buffer capacity, a solution should have large concentrations of both buffer components. If we know that the equilibrium concentration of h+ in a 1. 0m hf solution is 2. 7 x 10-2 m, and the percent dissociation of hf is 2. 7%. Calculate [h+] and the percent dissociation of hf in a solution containing 1. 0 m. Hf (ka = 7. 2 x 10-4) and 1. 0 m naf.