2
answers
0
watching
477
views
28 Sep 2019
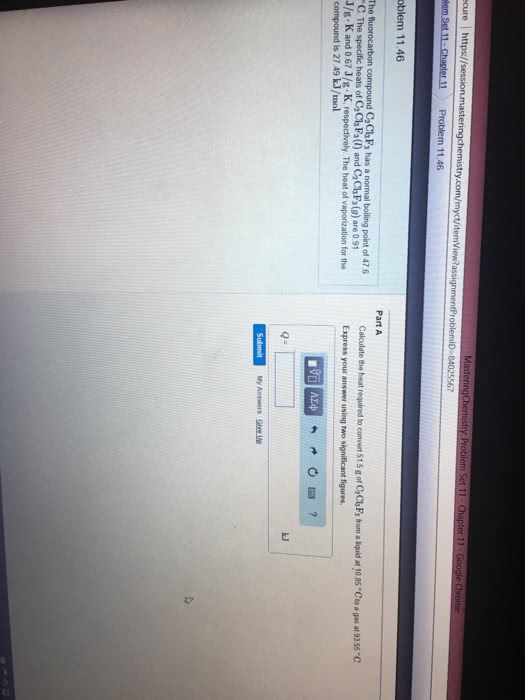
Sulfur dioxide is produced in enormous amounts for sulfuric acid production. It metls at -73.0 degrees Celsius and boils at -10.0 degrees celsius. Its change enthalpy (fusion) is 8.619 kJ/mol, and its change in enthalpy (vaporization) is 25.73 kJ/mol. The specific heat capacities of the liquid and gas are 0.995 J/g.K and 0.622J/g.K, respectively. How much heat is required to convert 2.750kg of solid SO2 at the metling point to a gas at 60.0 degrees Celsius?
Sulfur dioxide is produced in enormous amounts for sulfuric acid production. It metls at -73.0 degrees Celsius and boils at -10.0 degrees celsius. Its change enthalpy (fusion) is 8.619 kJ/mol, and its change in enthalpy (vaporization) is 25.73 kJ/mol. The specific heat capacities of the liquid and gas are 0.995 J/g.K and 0.622J/g.K, respectively. How much heat is required to convert 2.750kg of solid SO2 at the metling point to a gas at 60.0 degrees Celsius?
Read by 1 person
9 Jan 2023
Collen VonLv2
28 Sep 2019
Already have an account? Log in