2
answers
0
watching
2,297
views
28 Sep 2019
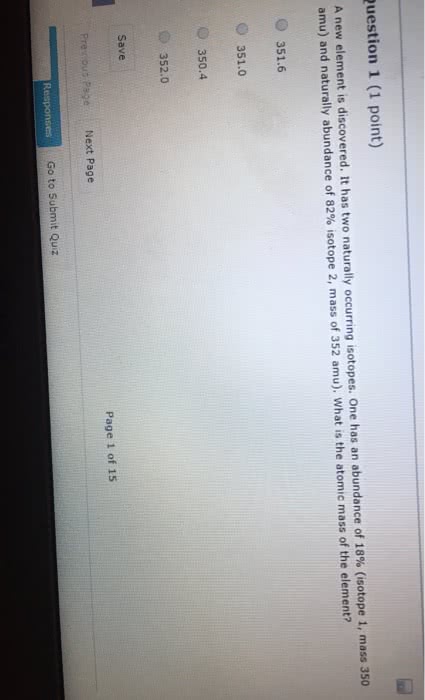
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu and a relative abundance of 42.6%.
1.Find the atomic mass of this element
Express your answer using four significant figures.
2. By comparison to the periodic table, identify it.
Express your answer as a chemical symbol.
An element has two naturally occurring isotopes. Isotope 1 has a mass of 120.9038 amu and a relative abundance of 57.4%, and isotope 2 has a mass of 122.9042 amu and a relative abundance of 42.6%.
1.Find the atomic mass of this element
Express your answer using four significant figures.
2. By comparison to the periodic table, identify it.
Express your answer as a chemical symbol.
shitalbhusare12Lv10
26 Mar 2022
Nelly StrackeLv2
28 Sep 2019
Already have an account? Log in