1
answer
0
watching
421
views
6 Nov 2019
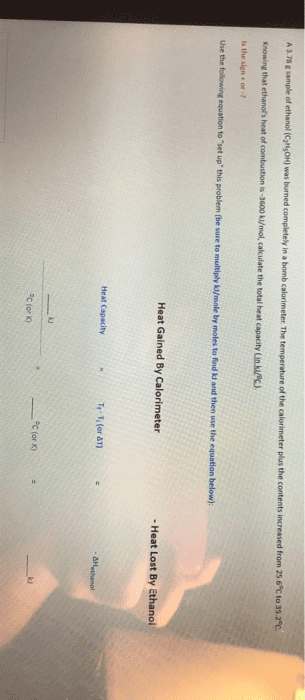
A sample of ethanol (C2H5OH) was burned completely in a bomb calorimeter whose total heat capacity is 30.75 kJ/oC. The temperature of the calorimeter plus the contents increased from 25.6oC to 32.7oC.
Knowing that ethanol's heat of combustion is -3330 kJ/mol, calculate the mass of the sample (in g).
A sample of ethanol (C2H5OH) was burned completely in a bomb calorimeter whose total heat capacity is 30.75 kJ/oC. The temperature of the calorimeter plus the contents increased from 25.6oC to 32.7oC.
Knowing that ethanol's heat of combustion is -3330 kJ/mol, calculate the mass of the sample (in g).
Sixta KovacekLv2
15 May 2019