2
answers
0
watching
1,033
views
17 Nov 2019
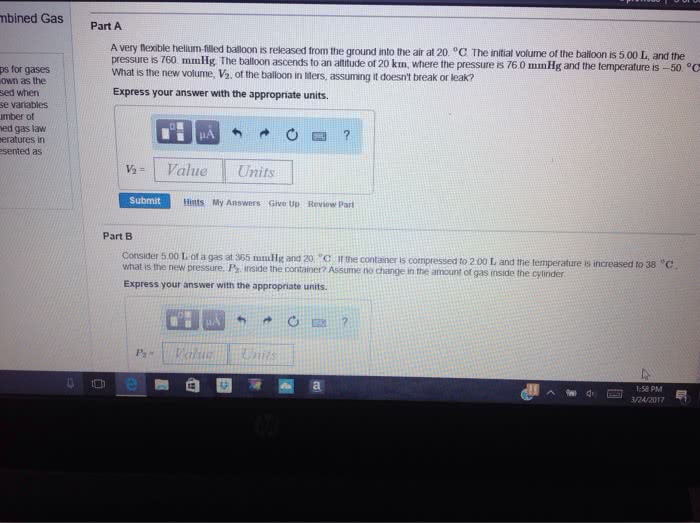
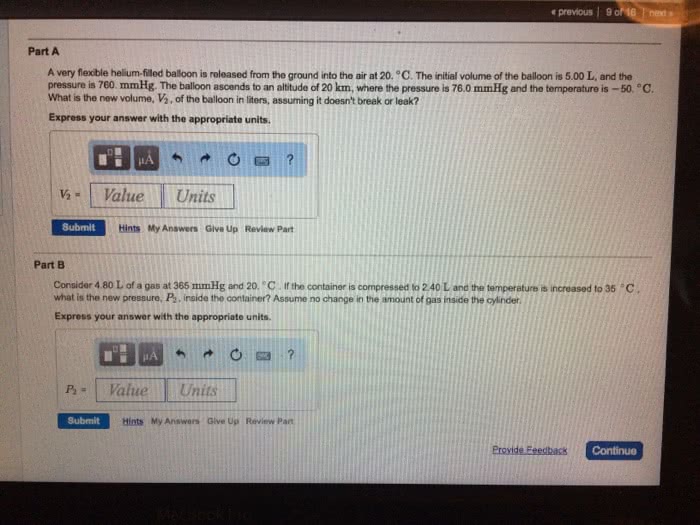
A) What pressure would it take to compress 150. L of helium gas initially at 1.00 atm into a 2.00 L tank at constant temperature?
Express your answer with the appropriate units.
B)A balloon filled with 2.00 L of helium initially at 1.55 atm of pressure rises into the atmosphere. When the surrounding pressure reaches 490. mmHg, the balloon will burst. If 1 atm = 760. mmHg, what volume will the balloon occupy in the instant before it bursts?
Express your answer with the appropriate units.
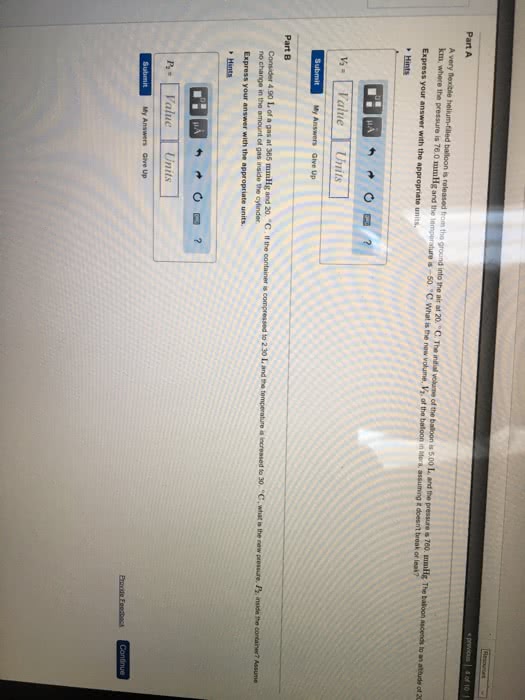
A) What pressure would it take to compress 150. L of helium gas initially at 1.00 atm into a 2.00 L tank at constant temperature?
Express your answer with the appropriate units.
B)A balloon filled with 2.00 L of helium initially at 1.55 atm of pressure rises into the atmosphere. When the surrounding pressure reaches 490. mmHg, the balloon will burst. If 1 atm = 760. mmHg, what volume will the balloon occupy in the instant before it bursts?
Express your answer with the appropriate units.
Jean KeelingLv2
23 Jun 2019
Already have an account? Log in