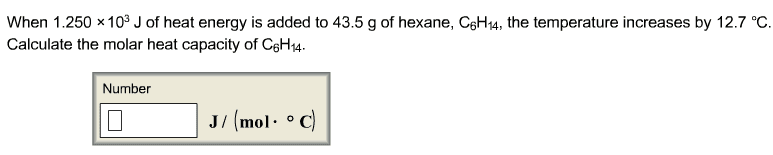
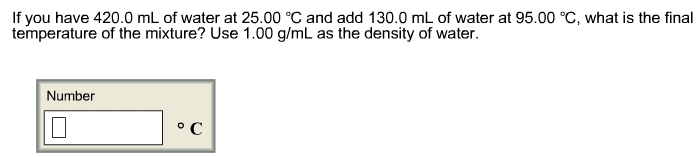1. A 88.3 g sample of metal at 95.24 oCis added to 35.10 g of water that is initially at 17.27oC.The final temperature of both the water and the metal is29.20oC.The specific heat of water is 4.184 J/(goC).Calculate the specific heat of the metal.
2. A 56.4 g sample of Al at 93.3 oCis added to 173.7 g of water that is initially at 27.0oC.The specific heat of Al and water are 0.90 J/(goC)and 4.184 J/(goC),respectively. Calculate the equilibrium temperature.
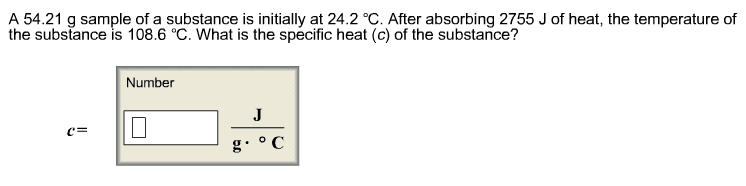
A 66 mL solution of a dilute AgNO3solution is added to 88 mL of a base solution in a coffee-cupcalorimeter. As AgOH (s) precipitates, the temperature of thesolution increases from 23.35 oCto 25.65 oC.Assuming the mixture has the same specific heat(4.184J/goC)and density (1.00 g/cm3or 1.00 g/mL)as water, calculate the heat (in J) transferred to thesurroundings, qsurr.
1. A 88.3 g sample of metal at 95.24 oCis added to 35.10 g of water that is initially at 17.27oC.The final temperature of both the water and the metal is29.20oC.The specific heat of water is 4.184 J/(goC).Calculate the specific heat of the metal.
2. A 56.4 g sample of Al at 93.3 oCis added to 173.7 g of water that is initially at 27.0oC.The specific heat of Al and water are 0.90 J/(goC)and 4.184 J/(goC),respectively. Calculate the equilibrium temperature.
A 66 mL solution of a dilute AgNO3solution is added to 88 mL of a base solution in a coffee-cupcalorimeter. As AgOH (s) precipitates, the temperature of thesolution increases from 23.35 oCto 25.65 oC.Assuming the mixture has the same specific heat(4.184J/goC)and density (1.00 g/cm3or 1.00 g/mL)as water, calculate the heat (in J) transferred to thesurroundings, qsurr.
For unlimited access to Homework Help, a Homework+ subscription is required.