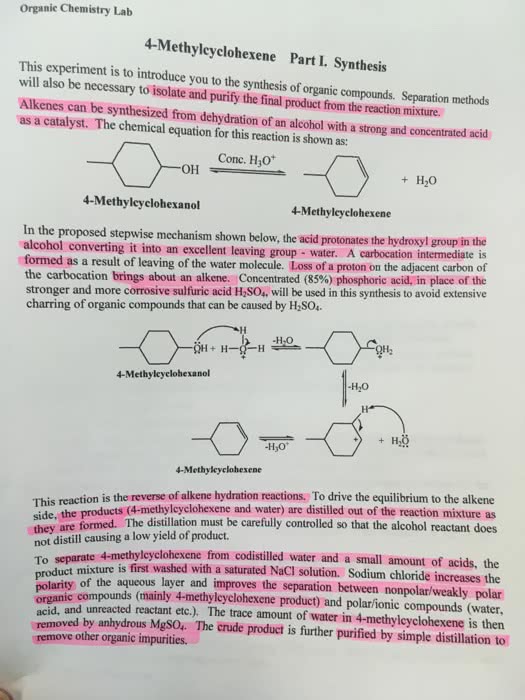
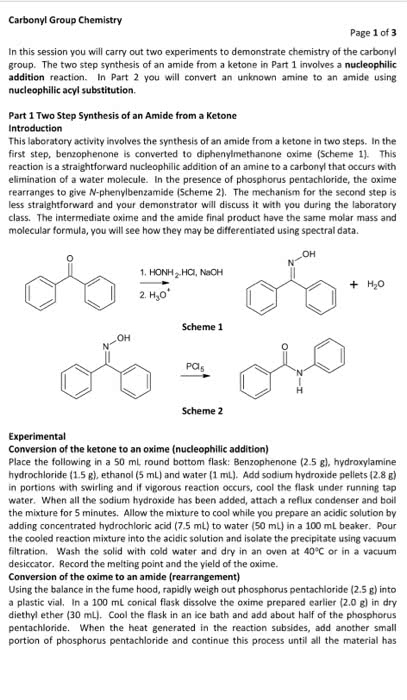
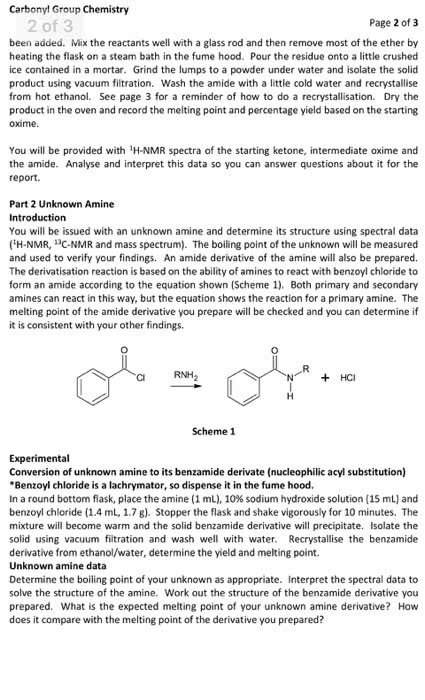
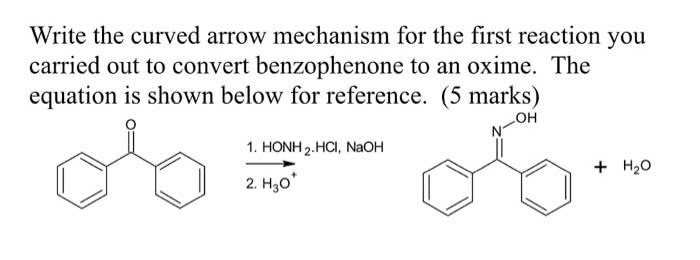
I want another 600 word discussion on 3-methyl-3-pentanol, here is the attached example if you need an idea. I don't want it to be the same.
"Discussion:
In this experiment, the 3-methyl-3-pentanol solution was being used in the dehydration process. The alcohol was a five carbons aliphatic hydrocarbon which one of the hydrogen atoms, H was substituted by one hydroxyl group, OH-. Due to the low melting point, the pentanol appear in liquid form at room temperature. The dehydration process of alcohol converted pentanol which the hydroxyl group, OH-was removed to become pentene. Pantene was a five carbons aliphatic hydrocarbon with a single double bond in the molecule. The solution was added with concentrated phosphoric acid in a 5-ml vial and was mixed together by swirling. The phosphoric acid was added as catalyze as such increased the rate of reaction in dehydration without affected the particular chemical reaction. The spin vain added into the solution in order to prevent over boiling of the solution.
The heating of mixture was carried out in a fractional distillation apparatus. As the mixture was heated, the alkene and water were produced as the products in the reaction. Besides, the side product and impurities of the reaction was being produced at the same time. The temperature of mixture when heating was fluctuated. During the temperature 80°C, the temperature of mixture dropped suddenly by 2°C and the temperature remained at 78°C constantly for few seconds. This was because some impurities or side products were being produced in the mixture which might had the boiling point of 78°C. The temperature of mixture dropped suddenly because the heat being was absorbed which used to break down the bonding of the side products. When the boiling point was reached, then temperature of mixture remained constant as the state of the side products are converted from liquid form to gaseous form. The temperature of mixture was increased until 107°C after all the side products were being converted.The temperature of mixture was reached to maximum at 107°C. This temperature was known as the activation temperature which the pentanol started to be dehydrated. In all the distillation process, some of the product was being lost since it washoled up in the apparatus which reduce the product yield. In order to maximize the yield, the mixture was continued to be heated at higher temperature range which more than the boiling point of pentanol. When the mixture was heated at 90°C-100°C, the water in the mixture pushed over the products into the receiving vial along the condenser. The products produced were collected in the same receiving vial.
Then, the receiving vial containing pentene, water and small amount of the impurities. Two layers liquid were presented in the receiving vial, one drop of distilled water was added into vial in order to determine the location of aqueous layer. Since the water droplet mixed with the lower layer, so the upper layer was determined as cloudy solution while the lower layer was aqueous layer. The upper cloudy layer was pentene with some impurities and water inside. The lower aqueous layer was removed and discarded. But, that is not easy to remove all the water in the receiving vial. So, anhydrous calcium chloride was being added. The purpose of adding of anhydrous calcium chloride was used to remove residual water in the organic solvent. It is known as drying agent in the organic solvent which are not dissolves in the solvent but drying the solvent. The calcium chloride clumped together with the water droplets as it solidified them. In another word, it reacted with water to form hydrates which was their preferred form when water was available. An excess drying agent should be used to ensure that all the water in solvent was removed. If the water remains in the materials collected, it could interfere with the analysis.Water had been successfully removed from the organic compound mixture, so it was very important to not reintroduce water into the mixture. The final distillation of unpurified 3-methyl-3-pentene must be done very carefully to obtain purified products. "
I want another 600 word discussion on 3-methyl-3-pentanol, here is the attached example if you need an idea. I don't want it to be the same.
"Discussion:
In this experiment, the 3-methyl-3-pentanol solution was being used in the dehydration process. The alcohol was a five carbons aliphatic hydrocarbon which one of the hydrogen atoms, H was substituted by one hydroxyl group, OH-. Due to the low melting point, the pentanol appear in liquid form at room temperature. The dehydration process of alcohol converted pentanol which the hydroxyl group, OH-was removed to become pentene. Pantene was a five carbons aliphatic hydrocarbon with a single double bond in the molecule. The solution was added with concentrated phosphoric acid in a 5-ml vial and was mixed together by swirling. The phosphoric acid was added as catalyze as such increased the rate of reaction in dehydration without affected the particular chemical reaction. The spin vain added into the solution in order to prevent over boiling of the solution.
The heating of mixture was carried out in a fractional distillation apparatus. As the mixture was heated, the alkene and water were produced as the products in the reaction. Besides, the side product and impurities of the reaction was being produced at the same time. The temperature of mixture when heating was fluctuated. During the temperature 80°C, the temperature of mixture dropped suddenly by 2°C and the temperature remained at 78°C constantly for few seconds. This was because some impurities or side products were being produced in the mixture which might had the boiling point of 78°C. The temperature of mixture dropped suddenly because the heat being was absorbed which used to break down the bonding of the side products. When the boiling point was reached, then temperature of mixture remained constant as the state of the side products are converted from liquid form to gaseous form. The temperature of mixture was increased until 107°C after all the side products were being converted.The temperature of mixture was reached to maximum at 107°C. This temperature was known as the activation temperature which the pentanol started to be dehydrated. In all the distillation process, some of the product was being lost since it washoled up in the apparatus which reduce the product yield. In order to maximize the yield, the mixture was continued to be heated at higher temperature range which more than the boiling point of pentanol. When the mixture was heated at 90°C-100°C, the water in the mixture pushed over the products into the receiving vial along the condenser. The products produced were collected in the same receiving vial.
Then, the receiving vial containing pentene, water and small amount of the impurities. Two layers liquid were presented in the receiving vial, one drop of distilled water was added into vial in order to determine the location of aqueous layer. Since the water droplet mixed with the lower layer, so the upper layer was determined as cloudy solution while the lower layer was aqueous layer. The upper cloudy layer was pentene with some impurities and water inside. The lower aqueous layer was removed and discarded. But, that is not easy to remove all the water in the receiving vial. So, anhydrous calcium chloride was being added. The purpose of adding of anhydrous calcium chloride was used to remove residual water in the organic solvent. It is known as drying agent in the organic solvent which are not dissolves in the solvent but drying the solvent. The calcium chloride clumped together with the water droplets as it solidified them. In another word, it reacted with water to form hydrates which was their preferred form when water was available. An excess drying agent should be used to ensure that all the water in solvent was removed. If the water remains in the materials collected, it could interfere with the analysis.Water had been successfully removed from the organic compound mixture, so it was very important to not reintroduce water into the mixture. The final distillation of unpurified 3-methyl-3-pentene must be done very carefully to obtain purified products. "