1
answer
0
watching
169
views
11 Dec 2019
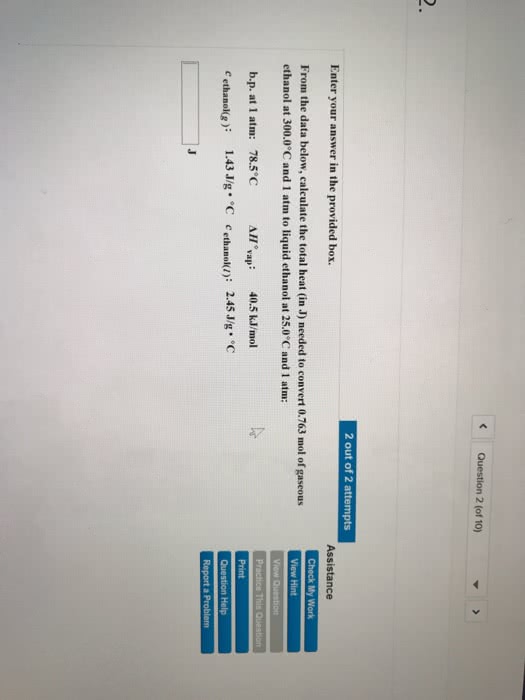
Enter your answer in the provided box. From the data below, calculate the total heat (in J) needed to convert 0.579 mol of gaseous ethanol at 300.0°C and 1 atm to liquid ethanol at 25.0°C and 1 atm: b.p. at 1 atm: 78.5°C ÎH o vap : 40.5 kJ/mol cgas: 1.43 J/g·°C cliquid: 2.45 J/g·°C
Enter your answer in the provided box. From the data below, calculate the total heat (in J) needed to convert 0.579 mol of gaseous ethanol at 300.0°C and 1 atm to liquid ethanol at 25.0°C and 1 atm: b.p. at 1 atm: 78.5°C ÎH o vap : 40.5 kJ/mol cgas: 1.43 J/g·°C cliquid: 2.45 J/g·°C
Jarrod RobelLv2
13 Dec 2019