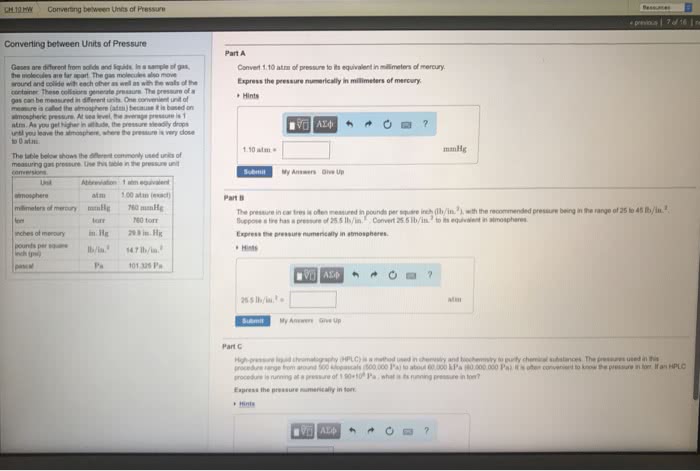
Converting between Units of Pressure Gases are different from solids and liquids. In a sample of gas, the molecules are far apart. The gas molecules also move around and collide with each other as well as with the walls of the container. These collisions generate pressure. The pressure of a gas can be measured in different units. One convenient unit of measure is called the atmosphere (atm) because it is based on atmospheric pressure. At sea level, the average pressure is 1 atm. As you get higher in altitude, the pressure steadily drops until you leave the atmosphere, where the pressure is very close to 0 atm. The table below shows the different commonly used units of measuring gas pressure. Use this table in the pressure unit conversions. Unit Abbreviation 1 atm equivalent atmosphere atm 1.00 atm (exact) millimeters of mercury mmHg 760 mmHg torr torr 760 torr inches of mercury in.Hg 29.9 in.Hg pounds per square inch (psi) lb/in.2 14.7 lb/in.2 pascal Pa 101,325 Pa.
Part C
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 5.81Ã108 Pa , what is its running pressure in torr?
Express the pressure numerically in torr.
Converting between Units of Pressure Gases are different from solids and liquids. In a sample of gas, the molecules are far apart. The gas molecules also move around and collide with each other as well as with the walls of the container. These collisions generate pressure. The pressure of a gas can be measured in different units. One convenient unit of measure is called the atmosphere (atm) because it is based on atmospheric pressure. At sea level, the average pressure is 1 atm. As you get higher in altitude, the pressure steadily drops until you leave the atmosphere, where the pressure is very close to 0 atm. The table below shows the different commonly used units of measuring gas pressure. Use this table in the pressure unit conversions. Unit Abbreviation 1 atm equivalent atmosphere atm 1.00 atm (exact) millimeters of mercury mmHg 760 mmHg torr torr 760 torr inches of mercury in.Hg 29.9 in.Hg pounds per square inch (psi) lb/in.2 14.7 lb/in.2 pascal Pa 101,325 Pa.
Part C
High-pressure liquid chromatography (HPLC) is a method used in chemistry and biochemistry to purify chemical substances. The pressures used in this procedure range from around 500 kilopascals (500,000 Pa) to about 60,000 kPa (60,000,000 Pa). It is often convenient to know the pressure in torr. If an HPLC procedure is running at a pressure of 5.81Ã108 Pa , what is its running pressure in torr?
Express the pressure numerically in torr.