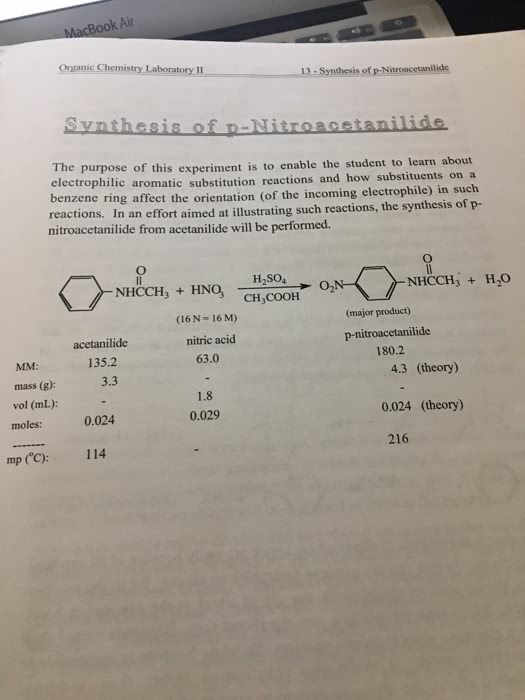
Question: For the following two experiments I have been asked to find at least 1 procedural change for each that could be made to improve the yield of my product. Please review the experiments and aid me in determining what this change might be. Thanks in advance!
Reduction of Benzophenone
1. In a 50 mL Erlenmeyer flask, dissolve 2.0 g benzophenone in 10 mL of methanol. Also add a stirbar to magnetically stir the solution.
2. Place your solution in an ice bath until the temperature has dropped to 10 C.
3. While your flask is in the ice bath, add 0.42 g of sodium borohydride, in small portions, over a 5 min period. The addition of sodium borohydride is exothermic, so you must take care to add it slowly to keep the temperature under control. This means adding the hydride in 5-6 portions, with about 1 min in between additions.
4. Once the addition of NaBH4 is complete and the vigorous bubbling has ceased, allow the solution to stir at room temperature for 10 minutes.
5. Carefully clamp the Erlenmeyer flask into a 80°C water bath and allow it to sit in the hot water bath for 2 minutes.
6. Remove the solution from the hot water bath and allow it to cool to room temperature and then cool it in an ice bath.
7. SLOWLY add 30 mL of cold water and then SLOWLY(dropwise) add 1.4 mL of 6M HCl. Use litmus paper to confirm that your solution is acidic. If your solution is not acidic, add more HCl until it is acidic. You should see the formation of a white precipitate at this point.
8. After adding the HCl, remove your solution from the ice bath and let it stir at room temperature for 20 minutes.
9. Collect the product by suction filtration, washing with 10-25 mL of cold water.
10. Allow the product to completely dry under suction and then record the crude weight.
11. Analyze the purity of your crude product by running a TLC. Dissolve a very small sample of your crude product and the benzophenone starting material in diethyl ether. Spot these samples on two separate lanes of your TLC place and develop the TLC pate with 9:1 petroleum ether: ethyl acetate.
12. If you are convinced that your crude sample is pure diphenylmethanol, record the final mass and run a melting point.
13. If you think some impurities are still present in your crude mixture, recrystallize your product with a minimal amount of hot hexanes. Review your notes from earlier in the semester if you donât remember the steps to run a recrystallization.
14. Record the mass and melting point of your purified diphenylmethanol and place it in a labeled vial to turn in to your instructor.
Oxidation of Diphenylmethanol
1. Place approximately 300-mL of hot (from the tap) water in a 400-mL beaker, add a boiling stone, and place it on a hot plate. Heat the water until it is 90-95 oC. It is important to keep the temperature just below 100 oC; thermal decomposition of the permanganate may occur at higher temperatures resulting in the release of toxic fumes.
2. While your water bath is heating, place 4.82 mmol of diphenylmethanol in a 10- or 25- mL round-bottomed flask.
3. Weigh out approximately 4 grams of the oxidant mixture of KMnO4/CuSO4â¢5H2O.
4. If the temperature of your water bath has reached 90-95 oC, combine the oxidant with the diphenylmethanol in your round-bottomed flask.
5. Vigorously stir the mixture with a microspatula for 5-10 min until the flask is warm to the touch. A color change from purple-blue to brown/black should be observed as you are stirring the solids. This is an indication that a reaction is occurring.
6. When the flask is warm, equip the reaction vessel with an air condenser and clamp it in the hot water bath. Be sure that the solid reaction mixture is below the surface of the water.
7. Heat the reaction for 1 h. Continue to monitor the temperature of the water; if the temperature increases close to 100 deg C, add a bit of cold water to keep the temperature 90-95 deg C and lower the heat setting on your hot plate.
8. After 1 h of heating, remove the flask from the hot water and allow it to cool to room temperature. Remove the reflux condenser.
9. Add 10 mL of hexanes to the solid and stir to mix. Remove the hexanes with a pipette and place it into a 25-mL Erlenmeyer flask. Repeat and combine the extracts.
10. Dry the combined hexane extracts with Na2SO4; decant/filter the hexanes into a preweighed 25-mL Erlenmeyer flask; and evaporate the hexanes using a gentle stream of air.
11. Analyze the purity of your crude product by running a TLC. Make samples of the following for TLC: stock benzophenone, crude benzophenone, and benzhydrol. Spot these samples on three separate lanes of your TLC place and develop the TLC pate with 5:1 hexane/ethyl acetate.
12. If you are convinced that your crude sample is pure benzophenone, record the final mass and run a melting point.
13. If you think some impurities are still present in your crude mixture OR if your product is not a solid, recrystallize your product with a minimal amount of hot methanol or methanol/water. Review your notes from earlier in the semester if you donât remember the steps to run a recrystallization.
14. Record the mass and melting point of your purified benzophenone and place it in a labeled vial to turn in to your instructor.
Question: For the following two experiments I have been asked to find at least 1 procedural change for each that could be made to improve the yield of my product. Please review the experiments and aid me in determining what this change might be. Thanks in advance!
Reduction of Benzophenone
1. In a 50 mL Erlenmeyer flask, dissolve 2.0 g benzophenone in 10 mL of methanol. Also add a stirbar to magnetically stir the solution.
2. Place your solution in an ice bath until the temperature has dropped to 10 C.
3. While your flask is in the ice bath, add 0.42 g of sodium borohydride, in small portions, over a 5 min period. The addition of sodium borohydride is exothermic, so you must take care to add it slowly to keep the temperature under control. This means adding the hydride in 5-6 portions, with about 1 min in between additions.
4. Once the addition of NaBH4 is complete and the vigorous bubbling has ceased, allow the solution to stir at room temperature for 10 minutes.
5. Carefully clamp the Erlenmeyer flask into a 80°C water bath and allow it to sit in the hot water bath for 2 minutes.
6. Remove the solution from the hot water bath and allow it to cool to room temperature and then cool it in an ice bath.
7. SLOWLY add 30 mL of cold water and then SLOWLY(dropwise) add 1.4 mL of 6M HCl. Use litmus paper to confirm that your solution is acidic. If your solution is not acidic, add more HCl until it is acidic. You should see the formation of a white precipitate at this point.
8. After adding the HCl, remove your solution from the ice bath and let it stir at room temperature for 20 minutes.
9. Collect the product by suction filtration, washing with 10-25 mL of cold water.
10. Allow the product to completely dry under suction and then record the crude weight.
11. Analyze the purity of your crude product by running a TLC. Dissolve a very small sample of your crude product and the benzophenone starting material in diethyl ether. Spot these samples on two separate lanes of your TLC place and develop the TLC pate with 9:1 petroleum ether: ethyl acetate.
12. If you are convinced that your crude sample is pure diphenylmethanol, record the final mass and run a melting point.
13. If you think some impurities are still present in your crude mixture, recrystallize your product with a minimal amount of hot hexanes. Review your notes from earlier in the semester if you donât remember the steps to run a recrystallization.
14. Record the mass and melting point of your purified diphenylmethanol and place it in a labeled vial to turn in to your instructor.
Oxidation of Diphenylmethanol
1. Place approximately 300-mL of hot (from the tap) water in a 400-mL beaker, add a boiling stone, and place it on a hot plate. Heat the water until it is 90-95 oC. It is important to keep the temperature just below 100 oC; thermal decomposition of the permanganate may occur at higher temperatures resulting in the release of toxic fumes.
2. While your water bath is heating, place 4.82 mmol of diphenylmethanol in a 10- or 25- mL round-bottomed flask.
3. Weigh out approximately 4 grams of the oxidant mixture of KMnO4/CuSO4â¢5H2O.
4. If the temperature of your water bath has reached 90-95 oC, combine the oxidant with the diphenylmethanol in your round-bottomed flask.
5. Vigorously stir the mixture with a microspatula for 5-10 min until the flask is warm to the touch. A color change from purple-blue to brown/black should be observed as you are stirring the solids. This is an indication that a reaction is occurring.
6. When the flask is warm, equip the reaction vessel with an air condenser and clamp it in the hot water bath. Be sure that the solid reaction mixture is below the surface of the water.
7. Heat the reaction for 1 h. Continue to monitor the temperature of the water; if the temperature increases close to 100 deg C, add a bit of cold water to keep the temperature 90-95 deg C and lower the heat setting on your hot plate.
8. After 1 h of heating, remove the flask from the hot water and allow it to cool to room temperature. Remove the reflux condenser.
9. Add 10 mL of hexanes to the solid and stir to mix. Remove the hexanes with a pipette and place it into a 25-mL Erlenmeyer flask. Repeat and combine the extracts.
10. Dry the combined hexane extracts with Na2SO4; decant/filter the hexanes into a preweighed 25-mL Erlenmeyer flask; and evaporate the hexanes using a gentle stream of air.
11. Analyze the purity of your crude product by running a TLC. Make samples of the following for TLC: stock benzophenone, crude benzophenone, and benzhydrol. Spot these samples on three separate lanes of your TLC place and develop the TLC pate with 5:1 hexane/ethyl acetate.
12. If you are convinced that your crude sample is pure benzophenone, record the final mass and run a melting point.
13. If you think some impurities are still present in your crude mixture OR if your product is not a solid, recrystallize your product with a minimal amount of hot methanol or methanol/water. Review your notes from earlier in the semester if you donât remember the steps to run a recrystallization.
14. Record the mass and melting point of your purified benzophenone and place it in a labeled vial to turn in to your instructor.