1
answer
0
watching
1,903
views
13 Dec 2019
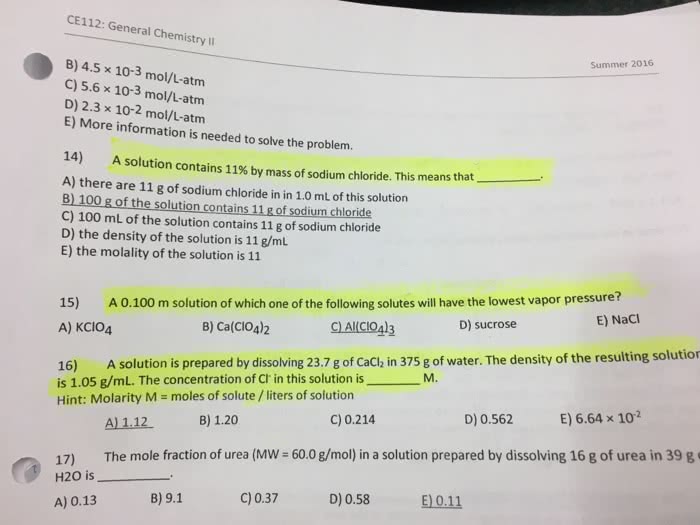
Which statement about hydrogen bonding is true?a) Hydrogen bonding is the intermolecular attractive forces between two hydrogen atoms in solution.b) The hydrogen bonding capabilities of water molecules cause CH3CH2CH2CH3 to be more soluble in water than CH3OH.c) Hydrogen bonding of solvent molecules with a solute will not affect the solubility of the solute.d) Hydrogen bonding interactions between molecules are stronger than the covalent bonds within the molecule.e) Hydrogen bonding arises from the dipole moment created by the unequal sharing of electrons within certain covalent bonds within a molecule.
Which statement about hydrogen bonding is true?a) Hydrogen bonding is the intermolecular attractive forces between two hydrogen atoms in solution.b) The hydrogen bonding capabilities of water molecules cause CH3CH2CH2CH3 to be more soluble in water than CH3OH.c) Hydrogen bonding of solvent molecules with a solute will not affect the solubility of the solute.d) Hydrogen bonding interactions between molecules are stronger than the covalent bonds within the molecule.e) Hydrogen bonding arises from the dipole moment created by the unequal sharing of electrons within certain covalent bonds within a molecule.
1
answer
0
watching
1,903
views
For unlimited access to Homework Help, a Homework+ subscription is required.
Verified Answer
Elin HesselLv2
17 Dec 2019
Joey Tang
Bachelor’s Degree in Chemistry from McMaster University21 May 2020
Answer verification
This is a step by step verification of the answer by our certified expert.
Subscribe to our livestream channel for more helpful videos.
Related textbook solutions
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232