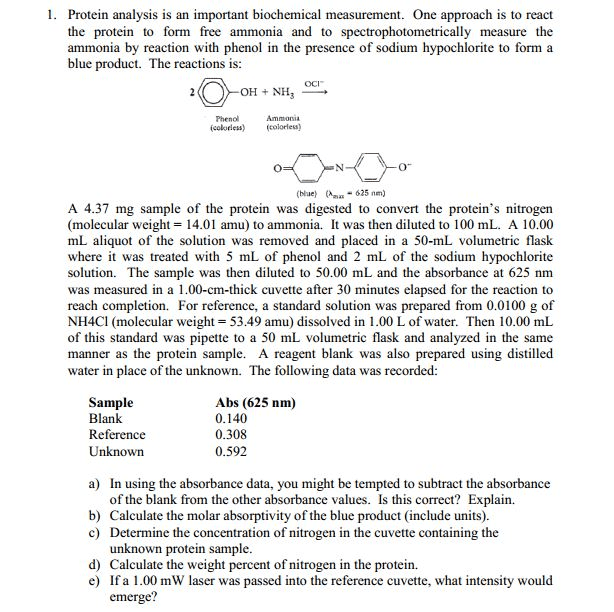
Treatment of ammonia with phenol in the presence of hypochlorite yields indophenol, a blue product absorbing light at 625 nm, which can be used for the spectrophotometric determination of ammonia.
To determine the ammonia concentration in a sample of lake water, you mix 10.0 mL of lake water with 5 mL of phenol solution and 2 mL of sodium hypochlorite solution and dilute to 25.0 mL in a volumetric flask (sample A). To a second 10.0 mL solution of lake water you add 5 mL of phenol, 2 mL of sodium hypochlorite, and 2.50 mL of a 5.50 Ã 10â4 M ammonia solution and dilute to 25.0 mL (sample B). As a reagent blank, you mix 10.0 mL of distilled water with 5 mL of phenol, 2 mL of sodium hypochlorite and dilute to 25.0 mL (sample C). You measure the following absorbances using a 1.00 cm cuvet:
Sample Absorbance(625 nm) A .385 B .632 C .045
What is the molar absorptivity (ε) of the indophenol product, and what is the concentration of ammonia in the lake water?
Treatment of ammonia with phenol in the presence of hypochlorite yields indophenol, a blue product absorbing light at 625 nm, which can be used for the spectrophotometric determination of ammonia.
To determine the ammonia concentration in a sample of lake water, you mix 10.0 mL of lake water with 5 mL of phenol solution and 2 mL of sodium hypochlorite solution and dilute to 25.0 mL in a volumetric flask (sample A). To a second 10.0 mL solution of lake water you add 5 mL of phenol, 2 mL of sodium hypochlorite, and 2.50 mL of a 5.50 Ã 10â4 M ammonia solution and dilute to 25.0 mL (sample B). As a reagent blank, you mix 10.0 mL of distilled water with 5 mL of phenol, 2 mL of sodium hypochlorite and dilute to 25.0 mL (sample C). You measure the following absorbances using a 1.00 cm cuvet:
| Sample | Absorbance(625 nm) |
| A | .385 |
| B | .632 |
| C | .045 |
What is the molar absorptivity (ε) of the indophenol product, and what is the concentration of ammonia in the lake water?