Part A: A calorimeter contains 17.0 mL of water at 12.0 ?C . When 2.50 g of X (a substance with a molar mass of 42.0 g/mol ) is added, it dissolves via the reaction
X(s)+H2O(l)?X(aq)
and the temperature of the solution increases to 25.5 ?C .
Calculate the enthalpy change, ?H, for this reaction per mole of X.
Assume that the specific heat of the resulting solution is equal to that of water [4.18 J/(g??C)], that density of water is 1.00 g/mL, and that no heat is lost to the calorimeter itself, nor to the surroundings.
Express the change in enthalpy in kilojoules per mole to three significant figures.
Part B: Consider the reaction
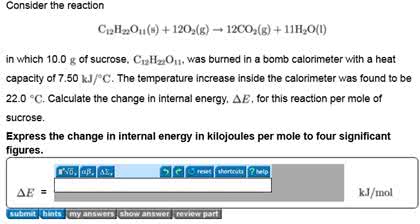
C12H22O11(s)+12O2(g)?12CO2(g)+11H2O(l)
in which 10.0 g of sucrose, C12H22O11, was burned in a bomb calorimeter with a heat capacity of 7.50 kJ/?C. The temperature increase inside the calorimeter was found to be 22.0 ?C. Calculate the change in internal energy, ?E, for this reaction per mole of sucrose.
Express the change in internal energy in kilojoules per mole to three significant figures.
Part A: A calorimeter contains 17.0 mL of water at 12.0 ?C . When 2.50 g of X (a substance with a molar mass of 42.0 g/mol ) is added, it dissolves via the reaction
X(s)+H2O(l)?X(aq)
and the temperature of the solution increases to 25.5 ?C .
Calculate the enthalpy change, ?H, for this reaction per mole of X.
Assume that the specific heat of the resulting solution is equal to that of water [4.18 J/(g??C)], that density of water is 1.00 g/mL, and that no heat is lost to the calorimeter itself, nor to the surroundings.
Express the change in enthalpy in kilojoules per mole to three significant figures.
Part B: Consider the reaction
C12H22O11(s)+12O2(g)?12CO2(g)+11H2O(l)
in which 10.0 g of sucrose, C12H22O11, was burned in a bomb calorimeter with a heat capacity of 7.50 kJ/?C. The temperature increase inside the calorimeter was found to be 22.0 ?C. Calculate the change in internal energy, ?E, for this reaction per mole of sucrose.
Express the change in internal energy in kilojoules per mole to three significant figures.