1
answer
0
watching
172
views
18 Dec 2019
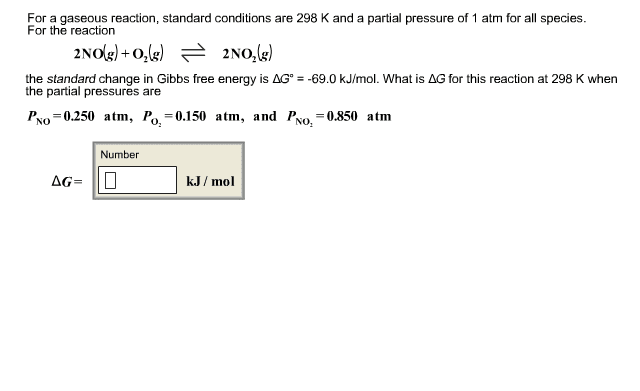
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species.
2NO(g)+O2->2NO2(g)
For the reactionthe standard change in Gibbs free energy is ÎG° = -69.0 kJ/mol. What is ÎG for this reaction at 298 K when the partial pressures are
P NO: .350 atm P O2: .500 atm
PNO2: .700 atm
delta G in kj/mol
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species.
2NO(g)+O2->2NO2(g)
For the reactionthe standard change in Gibbs free energy is ÎG° = -69.0 kJ/mol. What is ÎG for this reaction at 298 K when the partial pressures are
P NO: .350 atm P O2: .500 atm
PNO2: .700 atm
delta G in kj/mol
Reid WolffLv2
31 Dec 2019