1
answer
0
watching
437
views
21 Mar 2020
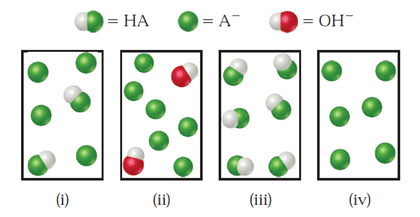
The following figure represents solutions at various stages of the titration of a weak acid, HA, with NaOH. (The Na+ ions and water molecules have been omitted for clarity.) To which of the following regions of the titration curve does each drawing correspond: (a) before addition of NaOH, (b) after addition of NaOH but before the equivalence point, (c) at the equivalence point, (d) after the equivalence point?

The following figure represents solutions at various stages of the titration of a weak acid, HA, with NaOH. (The Na+ ions and water molecules have been omitted for clarity.) To which of the following regions of the titration curve does each drawing correspond: (a) before addition of NaOH, (b) after addition of NaOH but before the equivalence point, (c) at the equivalence point, (d) after the equivalence point?
Irving HeathcoteLv2
23 May 2020