1
answer
0
watching
238
views
24 Mar 2020
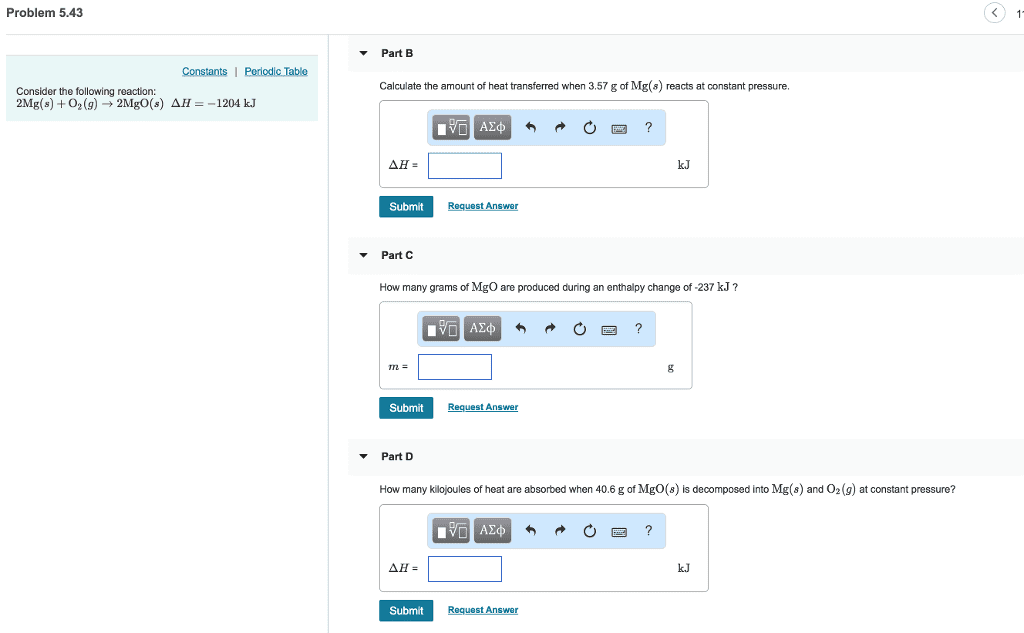
Consider the following reaction:
2 Mg(s) + O2(g)  2 MgO(s) ∆H = -1204 kJ
2 MgO(s) ∆H = -1204 kJ
(a) Is this reaction exothermic or endothermic?
(b) Calculate the amount of heat transferred when 3.55 g of Mg(s) reacts at constant pressure.
(c) How many grams of MgO are produced during an enthalpy change of –234 kJ?
(d) How many kilojoules of heat are absorbed when 40.3 g of MgO(s) is decomposed into Mg(s) and O2(g) at constant pressure?
Consider the following reaction:
2 Mg(s) + O2(g) 2 MgO(s) ∆H = -1204 kJ
(a) Is this reaction exothermic or endothermic?
(b) Calculate the amount of heat transferred when 3.55 g of Mg(s) reacts at constant pressure.
(c) How many grams of MgO are produced during an enthalpy change of –234 kJ?
(d) How many kilojoules of heat are absorbed when 40.3 g of MgO(s) is decomposed into Mg(s) and O2(g) at constant pressure?
Tod ThielLv2
25 May 2020