1
answer
1
watching
759
views
22 Apr 2020
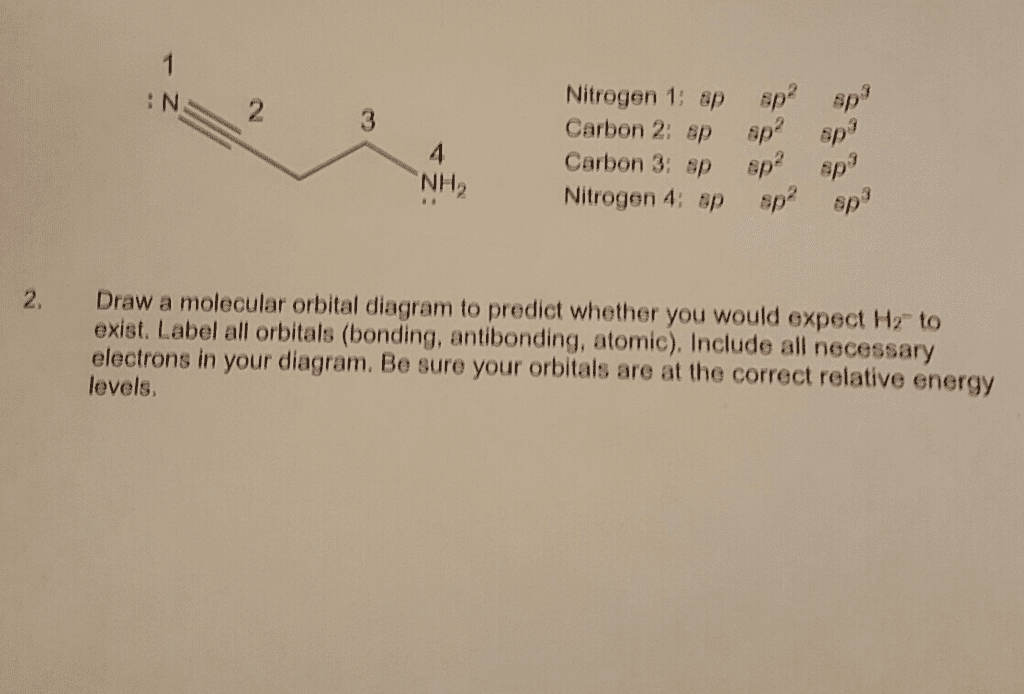
(a) Sketch the molecular orbitals of the H2– ion and draw its energy-level diagram. (b) Write the electron configuration of the ion in terms of its MOs. (c) Calculate the bond order in H2-. (d) Suppose that the ion is excited by light, so that an electron moves from a lower-energy to a higher-energy molecular orbital. Would you expect the excited-state H2- ion to be stable? (e) Which of the following statements about part (d) is correct: (i) The light excites an electron from a bonding orbital to an antibonding orbital, (ii) The light excites an electron from an antibonding orbital to a bonding orbital, or (iii) In the excited state there are more bonding electrons than antibonding electrons?
(a) Sketch the molecular orbitals of the H2– ion and draw its energy-level diagram. (b) Write the electron configuration of the ion in terms of its MOs. (c) Calculate the bond order in H2-. (d) Suppose that the ion is excited by light, so that an electron moves from a lower-energy to a higher-energy molecular orbital. Would you expect the excited-state H2- ion to be stable? (e) Which of the following statements about part (d) is correct: (i) The light excites an electron from a bonding orbital to an antibonding orbital, (ii) The light excites an electron from an antibonding orbital to a bonding orbital, or (iii) In the excited state there are more bonding electrons than antibonding electrons?
Reid WolffLv2
25 May 2020