
jed
John CayasUniversity of the Philippines Diliman
0 Followers
0 Following
7 Helped
Graduate of Bachelor of Science in Civil Engineering
jedLv1
29 Jun 2021
Answer: Decreasing the temperature and adding more will result in an increase ...
jedLv1
10 Jun 2021
Answer: The concentration of the complex ion will increase. Explanation: Le Ch...
jedLv1
2 Jun 2021
Fixing the smear is important because it prevents the sample from being lost i...
jedLv1
2 Jun 2021
Bond enthalpy (also known as bond energy) is defined as the amount of energy r...
jedLv1
2 Jun 2021
The equilibrium constant, Kc, is the ratio of the equilibrium concentrations o...
jedLv1
2 Jun 2021
In the problem, it is stated that under the condition , a good linear regressi...
jedLv1
2 Jun 2021
The Heisenberg Uncertainty Principle states that it is not possible to simulta...
jedLv1
2 Jun 2021
d. Some prokaryotes may use nitrate () as a terminal electron acceptor. Explan...
jedLv1
2 Jun 2021
Polypeptide chains can form tightly coiled helical structures that are stabili...
jedLv1
2 Jun 2021
Concentrated hydrobromic acid () is a very strong acid and in the presence of ...
jedLv1
2 Jun 2021
In the problem, we are asked to calculate the amount of heat required to conve...
jedLv1
2 Jun 2021
Electrical conductivity is a property of matter. It is a measure of the ease a...
jedLv1
2 Jun 2021
Since the initial and final pressures and temperatures are mostly given, we ca...
jedLv1
2 Jun 2021
All statements about globular and fibrous proteins and keratin are correct and...
jedLv1
2 Jun 2021
PART A: In the problem, two ions are to be titrated. When this is the case, th...
jedLv1
2 Jun 2021
PART A: The oxidation reaction makes use of oxygen to produce carbon dioxide a...
jedLv1
2 Jun 2021
For a van der Waals gas, Getting the derivative with respect to temperature, I...
jedLv1
2 Jun 2021
PART I: The pH and the ratio of the base concentration to the acid concentrati...
jedLv1
2 Jun 2021
For this problem, since there are two ions present in the solution, we can ass...
jedLv1
2 Jun 2021
We can calculate the molar concentration in the urine sample using the formula...
jedLv1
2 Jun 2021
The number of moles produced during any reaction can be determined using the m...
jedLv1
2 Jun 2021
PART A: The mole fraction of water in the inlet steam is . At constant pressur...
jedLv1
2 Jun 2021
Aggregation of protein occurs mostly when the protein is misfolded. Thus, aggr...
jedLv1
2 Jun 2021
In phenoxide, the oxide group will donate electron to the ring so that effecti...
jedLv1
2 Jun 2021
The amino acid sequence of an octapeptide can be determined from the amino aci...
jedLv1
2 Jun 2021
FIRST QUESTION: For the first question, we are asked to determine the degree o...
jedLv1
2 Jun 2021
It is essential for the dye molecules to be aromatic because aromatic compound...
jedLv1
2 Jun 2021
The isolation of pure amino acid is extremely difficult because of high temper...
jedLv1
2 Jun 2021
The concentration of a solution is a measure of the amount of solute that has ...
jedLv1
2 Jun 2021
For the first task in the problem, we are asked to write the major species at ...
jedLv1
2 Jun 2021
Chemical reactions are processes in which one or more substances are converted...
jedLv1
2 Jun 2021
Valence Shell Electron Pair Repulsion Theory (VSEPR) is a model used to predic...
jedLv1
2 Jun 2021
The concentration of a solution is a measure of the amount of solute that has ...
jedLv1
2 Jun 2021
We can determine the number of grams of copper metal that will be recovered fr...
jedLv1
2 Jun 2021
In this problem, we are asked to analyze the titration of ascorbic acid with i...
jedLv1
2 Jun 2021
A solution of any desired concentrations can be prepared by diluting a small v...
jedLv1
2 Jun 2021
The table from the given can be used as-is. The complete table set-up can be s...
jedLv1
2 Jun 2021
PART A: For this part of the problem, we would determine which of the followin...
jedLv1
2 Jun 2021
We can determine the empirical formula of the compound just by using the perce...
jedLv1
2 Jun 2021
In the problem, the quantity being asked is the molarity of that will be used ...





























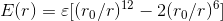
 and
and 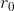 for carbon-carbon bonds are respectively
for carbon-carbon bonds are respectively 













































































