APS104H1 : 2011 APS104-MSE-Chapter03-NPK-L24[1].pdf
Document Summary
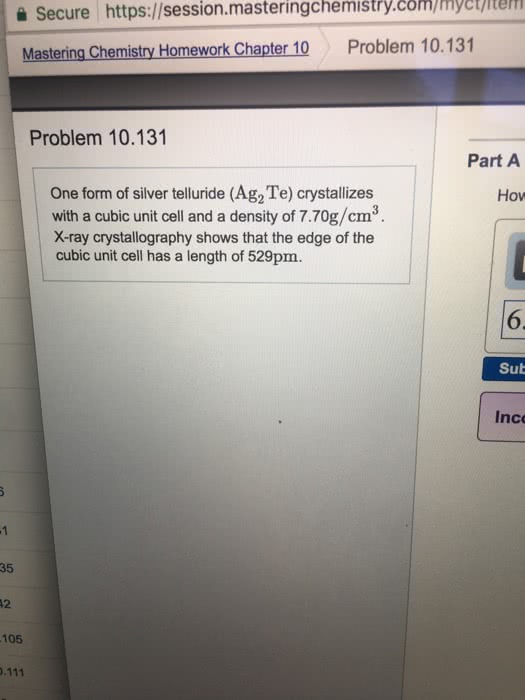
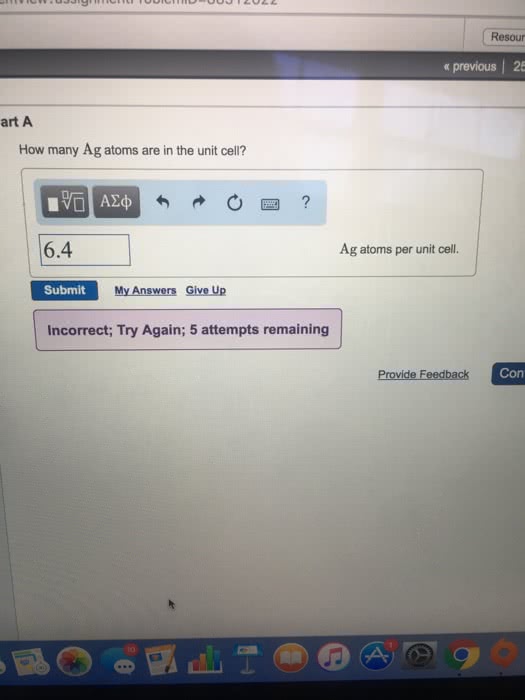
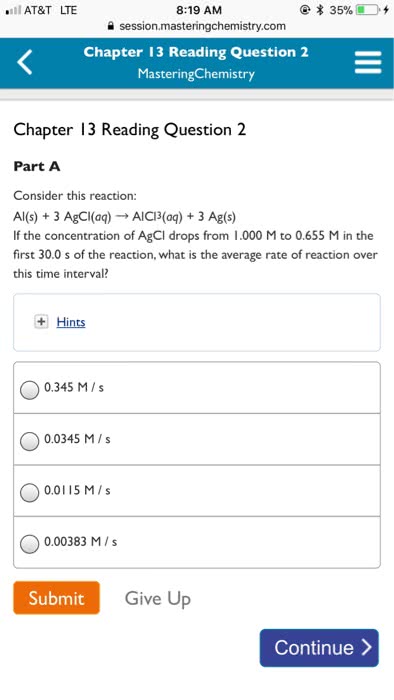
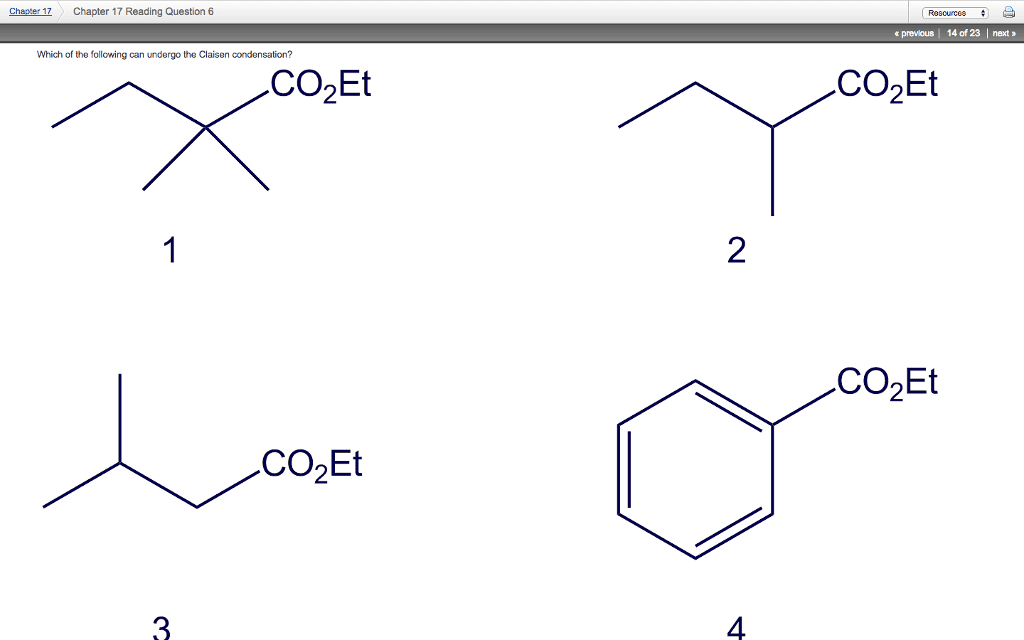
Polymorphism: two or more distinct crystal structures for the same material (allotropy/polymorphism) iron system liquid. Tetrahedral bonding of carbon: hardest material known, very high thermal conductivity, isotropic. Large single crystal-gem stones; small crystals used to grind/cut other materials. Diamond thin films: hard surface coatings used for cutting tools, medical devices, etc. Callister & rethwisch 3e. (0, 0, 0) (1/2, 1/2, 0) (1/4, 1/4, 1/4) Layered structure parallel hexagonal arrays of carbon atoms. Weak van der waal"s forces between layers. High thermal and electrical conductivity (in plane) Bad thermal and electrical conductivity (between plane, along c axis) Planes slide easily over one another -- good lubricant. Fullerenes and nanotubes: fullerenes spherical cluster of 60 carbon atoms, c60. Like a soccer ball: carbon nanotubes sheet of graphite rolled into a tube. Graphene: single sheet of graphite" (active area of current research) Unit cell: smallest repetitive volume which contains the complete lattice pattern of a crystal.