CHEM 1A03 Lecture Notes - Lecture 12: Bond-Dissociation Energy, Bond Order, Covalent Bond
207 views5 pages
Verified Note
3 Oct 2018
School
Department
Course
Professor
CHEM 1A03 verified notes
12/36View all
Document Summary
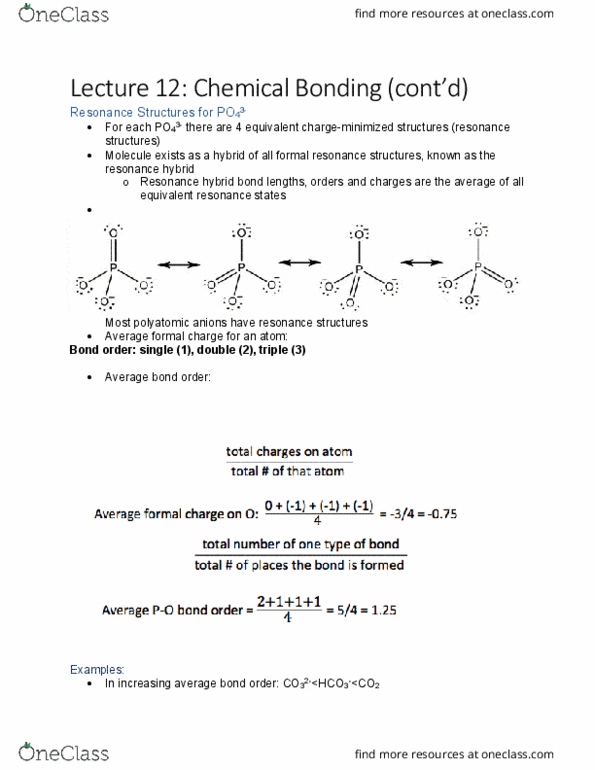
Most polyatomic anions have resonance structures: average formal charge for an atom: Bond order: single (1), double (2), triple (3: average bond order: , the terminal bonds are on 2 oxygens; one double bond and one. 2- is bigger, so it has a larger terminal bond order. Molecular shape: vsepr (valence shell electron pair repulsion) theory, aka gillespie-nyholm theory. [ron gillespie, mcmaster chemistry!: the one who first performed this theory, electron pairs repel one another, repulsion decreases in this order: Bonded pair-bonded pair: *note*: double bonds occupy slightly more space than a lone pair. 2 electron pairs: electron pair geometry - linear. 2 electron pairs: electron pair geometry - trigonal planar, lone pair of electrons also occupies space; which makes it a little bit less than. Dipole review: vectors from center of atom to the terminal atoms when added together all cancel out; then the atom has no dipole (all the terminal atoms are the same; ex.
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232