CHEM101 Lecture Notes - Lecture 5: Nonmetal, Effective Nuclear Charge, Atomic Number
CHEM101 verified notes
5/14View all
Document Summary
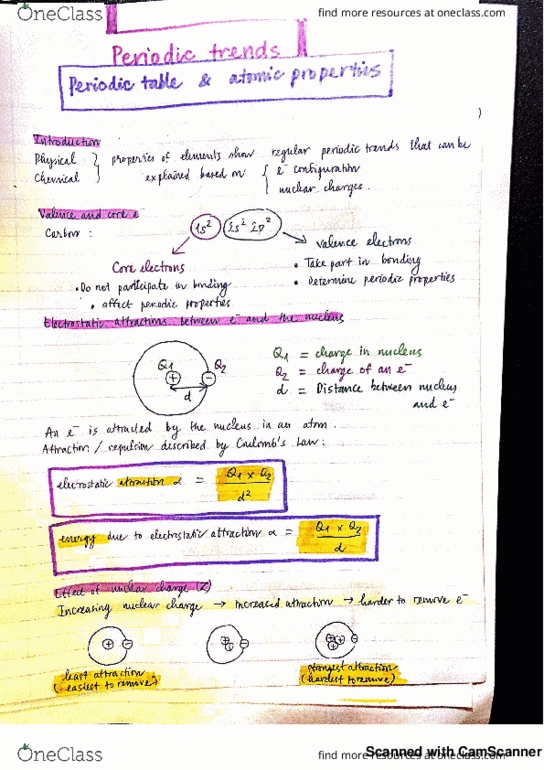
Introduction: the physical and chemical properties of the elements show regular periodic trends that can be explained based on the electron configuration and nuclear charges. Electrons in an atom can be classified as the valence and core electrons: In order to predict the periodic trends, it is important to understand the electrostatic forces of electrons in an atom. Do not participate in bonding but affect periodic properties. An electron is attracted by the nucleus in an atom. The attraction (repulsion if same charge) is described by the coulomb"s law: According to the coulomb"s law, increasing nuclear charge is expected to increase the attraction (energy) and so it is harder to remove the electron. Electron in the same shell as the electron of interest shields the nucleus attraction somewhat but not very effective. The effective nuclear charge (zeff) the actual nuclear charge the outermost electrons feel in an atom. Assume that shielding = 0. 2 for electron in the same orbital.