CHM136H1 Lecture Notes - Lecture 4: Diethyl Ether, Hexane, Pi Bond

34
CHM136H1 Full Course Notes
Verified Note
34 documents
Document Summary
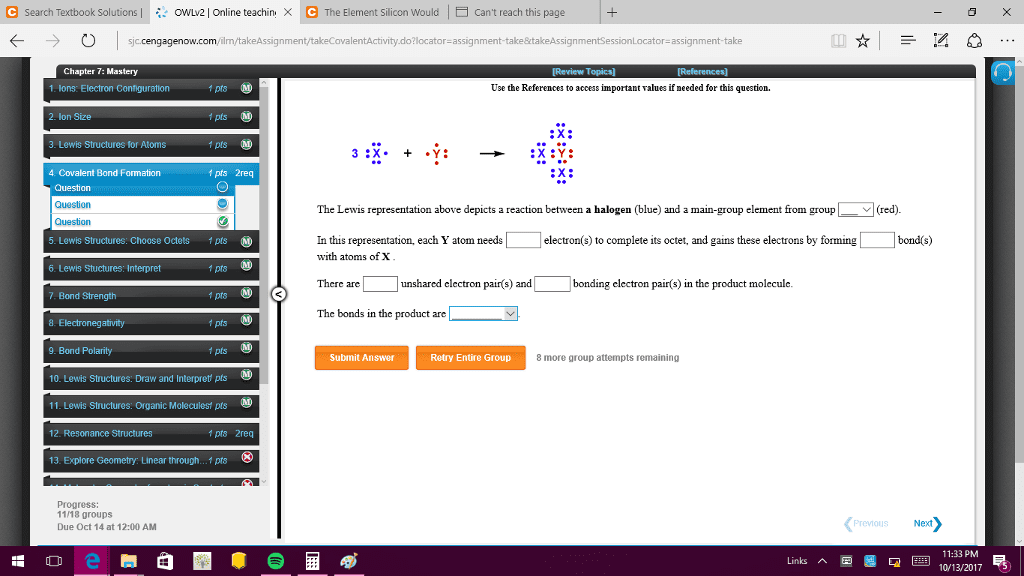
Chapter 2: non polar covalent bonds: atoms with similar en, when their difference is less than 0. 5, polar covalent, the electronegativity difference is less than 2. Carbon gains a partial positive charge, the electronegative atom gains a partial negative electron: ionic bond: the difference of en is over 2. Shifting of electrons in a bond in response to en of nearby atoms. The colors indicate : red (electron rich) blue (electron defecient) red : Oxygen pulls electron density away from the atom = blue. The arrows indicate the direction of the bond movement. Reversed polarity: when c is bonding to lithium, carbon is the more electronegative atom, the more electronegative atom carries the partial negative charge. Whole molecules often polar from vector summation of individual bond polarities and contributions from lone pair electrons. Strongly polar substances are soluble in polar solvents. Non polar substances insoluble in water, soluble in nonpolar solvents (hexane) (diethyl ether non polar)