CHE 131 Lecture Notes - Lecture 1: Density, Chemical Property, Physical Property
CHE 131 verified notes
1/46View all
Document Summary
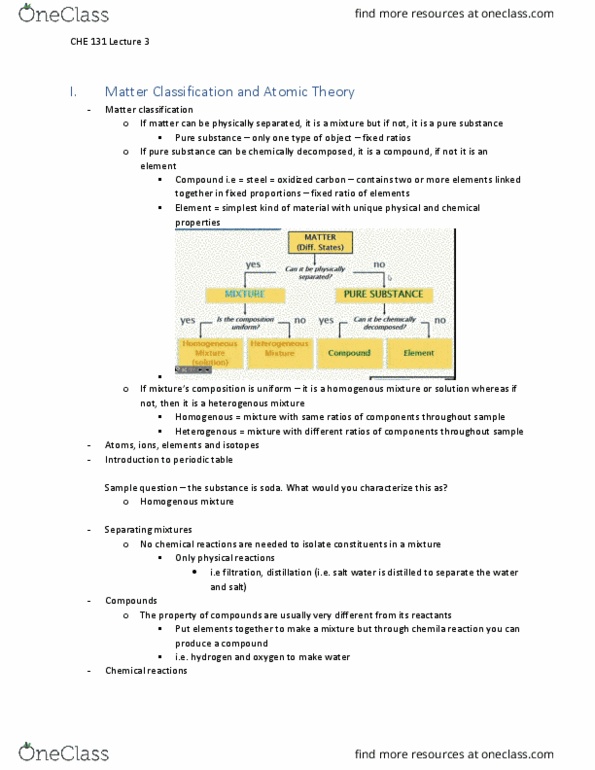
Measurements involve the change that matter undergoes and the energy associated with this change. Molecules, atoms and sub-atomic particles: major properties of matter is that it takes up space, has mass and attracts other matter with gravity. Mass fundamental property so it can only be defined indirectly. Characteristics that can be observed only when they react with another substance. My substance, combine - transformed into more than one new substance: hydrogen combines explosively with oxygen, physical properties. Characteristics that can be observed without changing into another substance: density, luster, hardness, color, pressure of air. A chemical property always involves a chemical reaction: physical property. Mass = density times volume volume = mass divided by density. Intensive property: a characteristic that is independent of the amount of substance present it is scale invariant, color, hardness, bouling point, melting point, conductivity, hardness,