CHM 111 Lecture Notes - Lecture 4: Polyatomic Ion, Molar Mass, Periodic Trends
CHM 111 verified notes
4/31View all
Document Summary
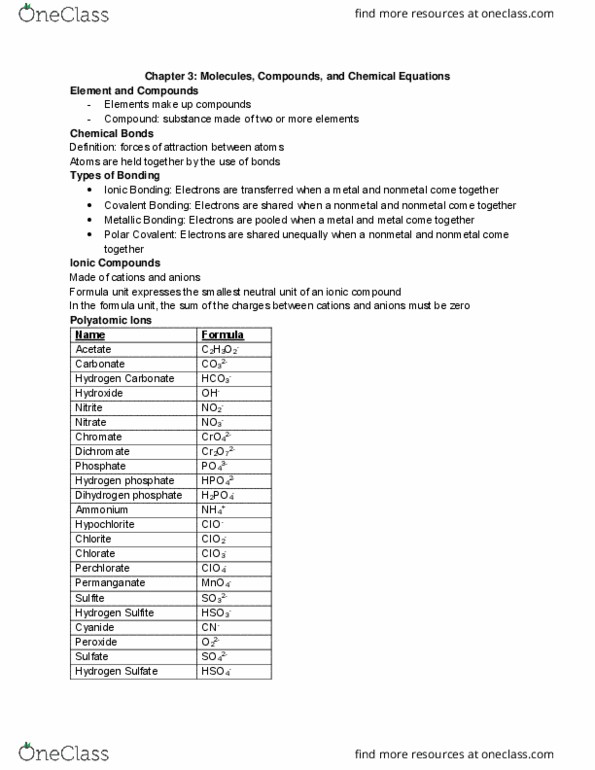
Atoms at start of reaction will be there at end of reaction (balanced) Electrons can transfer from one atom to the other. Ion: atom(s) with a positive or negative charge. When a neutral atom loses one or more electrons. Formed by metals: anion: negatively charged ion. When a neutral atom gains one or more electrons. Formed by nonmetals: monatomic ion: ion with one atom, polyatomic ion: ion with more than one atom. Mendeleev: elements are orders by their atomic mass, elements with similar properties are in the same column, periodic law arranges the elements by atomic mass. Metals: solid at room temperature (except for hg, conducts heat and electricity, loses electrons in reactions (cations, malleable, ductile, 75% of elements are metals. Nonmetals: poor conductors of heat and electricity, gains electrons in reactions (anions, solids are brittle. Metalloids: aka semiconductors, have properties of metals and nonmetals. Atomic mass: the average mass of an element.