CHEM 1127Q Lecture Notes - Lecture 7: Unified Atomic Mass Unit, Atomic Number, Carbon-12
CHEM 1127Q verified notes
7/22View all
Document Summary
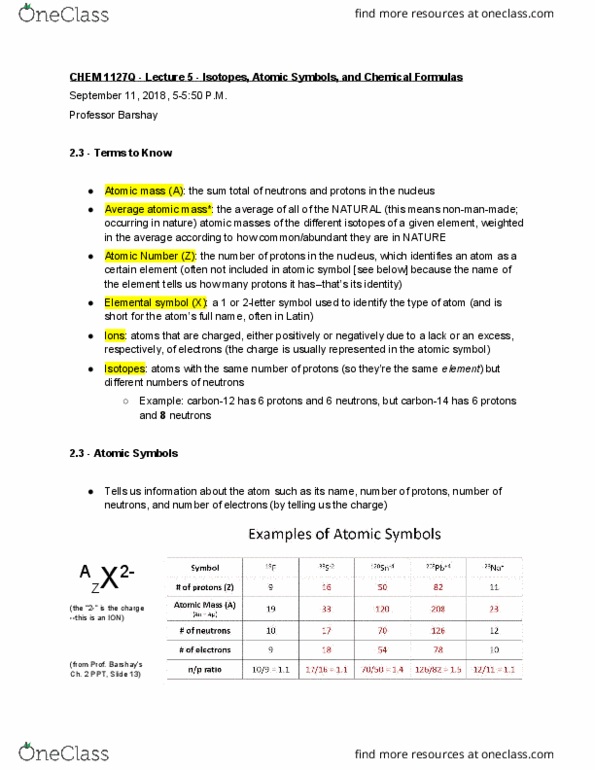
Chem 1127q - lecture 5 - isotopes, atomic symbols, and chemical formulas. Atomic mass (a) : the sum total of neutrons and protons in the nucleus. Average atomic mass* : the average of all of the natural (this means non-man-made; occurring in nature) atomic masses of the different isotopes of a given element, weighted in the average according to how common/abundant they are in nature. Elemental symbol (x) : a 1 or 2-letter symbol used to identify the type of atom (and is short for the atom"s full name, often in latin) Ions : atoms that are charged, either positively or negatively due to a lack or an excess, respectively, of electrons (the charge is usually represented in the atomic symbol) Isotopes : atoms with the same number of protons (so they"re the same element ) but different numbers of neutrons. Example: carbon-12 has 6 protons and 6 neutrons, but carbon-14 has 6 protons and 8 neutrons.