1
answer
0
watching
418
views
19 Nov 2019
1) What is the molarity of a 3.12 m solution of KCl dissolved in water, given that the solution has a density of 1.13 g/mL?
2)What is the concentration (ppm) of lead found in a water sample at the 3.29 x 10?5 M level?
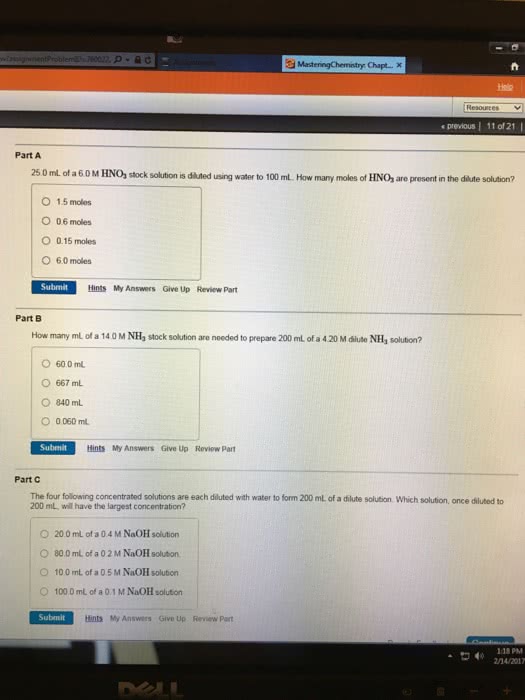
3) A 14.0 M solution of concentrated nitric acid has 20.0 mL withdrawn from it. The 20.0 mL sample is then diluted until the total volume of the resulting solution is 2.50 L. What is the concentration of the dilute solution?
4) To prepare a Cd2+ standard, 0.280 g of cadmium metal is first dissolved in 50 mL of concentrated HCl, followed by dilution to 1.00 L. This solution is followed by another dilution that reduces the concentration by 100-fold. What is the concentration of the resulting solution?
please!!!!!!!
1) What is the molarity of a 3.12 m solution of KCl dissolved in water, given that the solution has a density of 1.13 g/mL?
2)What is the concentration (ppm) of lead found in a water sample at the 3.29 x 10?5 M level?
3) A 14.0 M solution of concentrated nitric acid has 20.0 mL withdrawn from it. The 20.0 mL sample is then diluted until the total volume of the resulting solution is 2.50 L. What is the concentration of the dilute solution?
4) To prepare a Cd2+ standard, 0.280 g of cadmium metal is first dissolved in 50 mL of concentrated HCl, followed by dilution to 1.00 L. This solution is followed by another dilution that reduces the concentration by 100-fold. What is the concentration of the resulting solution?
please!!!!!!!
Beverley SmithLv2
15 Mar 2019