1
answer
0
watching
240
views
15 Jun 2020
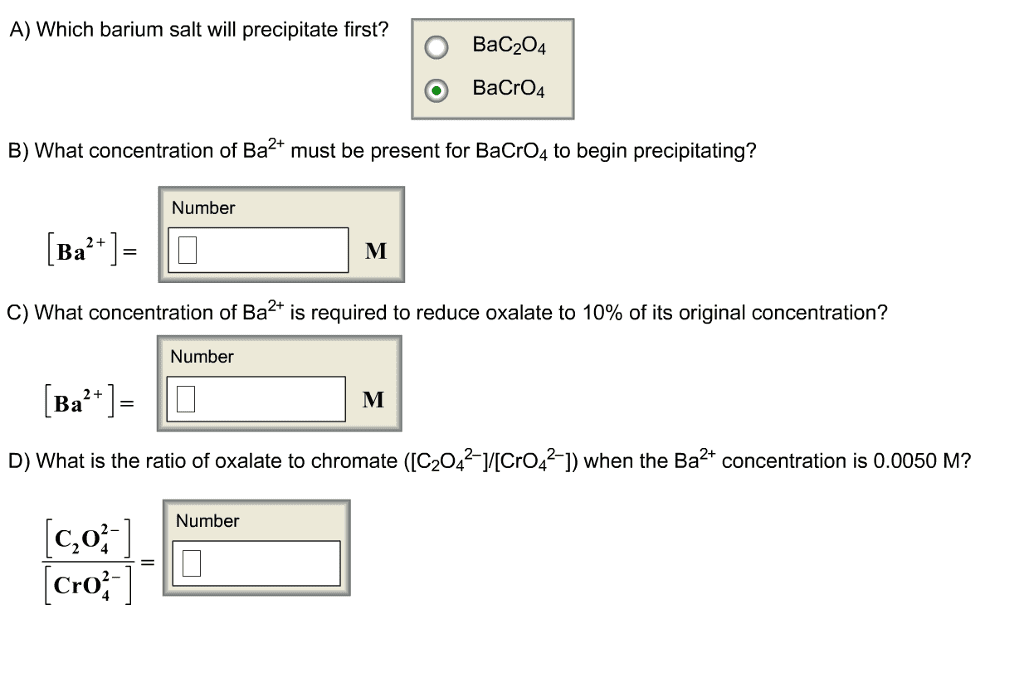
The Ksp for strontium chromate is 3.6×10−5 and the Ksp for barium chromate is 1.2×10−10. What concentration of potassium chromate will precipitate the maximum amount of either the barium or the strontium chromate from an equimolar 0.30 M solution of barium and strontium ions without precipitating the other?
The Ksp for strontium chromate is 3.6×10−5 and the Ksp for barium chromate is 1.2×10−10. What concentration of potassium chromate will precipitate the maximum amount of either the barium or the strontium chromate from an equimolar 0.30 M solution of barium and strontium ions without precipitating the other?
1 Sep 2020