CHEM 110 Lecture Notes - Lecture 6: Covalent Radius, Covalent Bond
25 views1 pages
Verified Note
29 Sep 2018
School
Department
Course
CHEM 110 verified notes
6/24View all
Document Summary
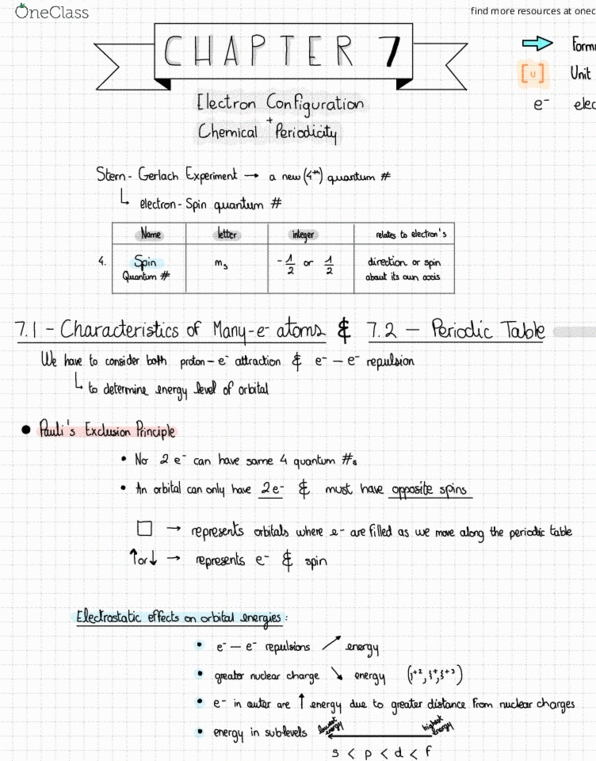
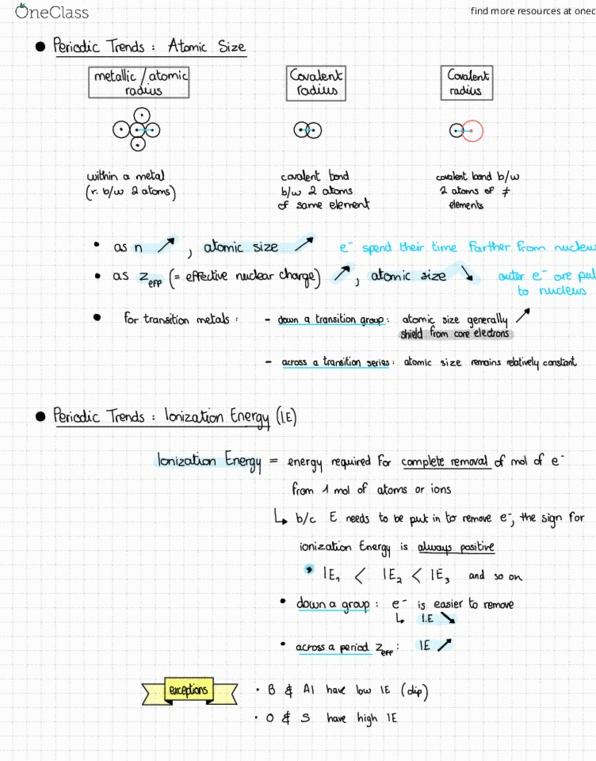
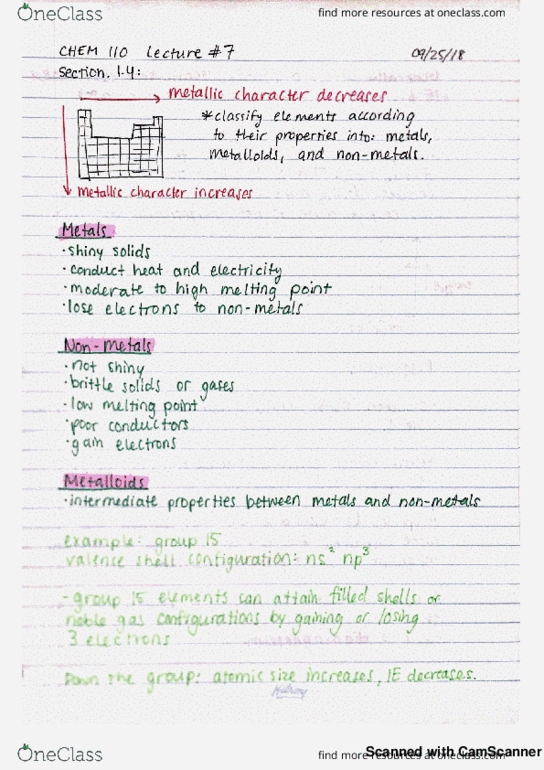
9 atomic siz bar outer e are pal d closer to nucleus down a transition group : atomic size shield from core. T. tt generally electrons across a transition series atomic size remains relatively constant: periodic trends. Ionizationenergy energy required for complete removal of mot of e. I ea to or ions needs atoms be put. Ie e is easier down a group : I. e m. across a in to remove e ; the sign for is alwayspositine and so on to remove exceptions go iron.
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers
Related textbook solutions
Chemistry: Structure and Properties
2 Edition,
Tro
ISBN: 9780134293936
Basic Chemistry
5 Edition,
Timberlake
ISBN: 9780134138046
Principles of Chemistry Molecular Approach
4th Edition,
Tro
ISBN: 9780134112831
Chemistry: Structure and Properties
2nd Edition,
Tro
ISBN: 9780134293936
Principles of Chemistry Molecular Approach
3rd Edition, 2014
Tro
ISBN: 9780321971944
Chemistry: A Molecular Approach
3rd Edition,
Tro
ISBN: 9780321809247
Chemistry: A Molecular Approach
5th Edition,
Tro
ISBN: 9780134874371
Principles of Chemistry: A Molecular Approach
4th Edition,
Tro
ISBN: 9780134895741
Chemistry: The Central Science
14th Edition, 2017
Brown
ISBN: 9780134414232