CHEM 120 Lecture Notes - Lecture 22: Intermolecular Force, Sodium Chloride, Molar Mass
CHEM 120 verified notes
22/28View all
Document Summary
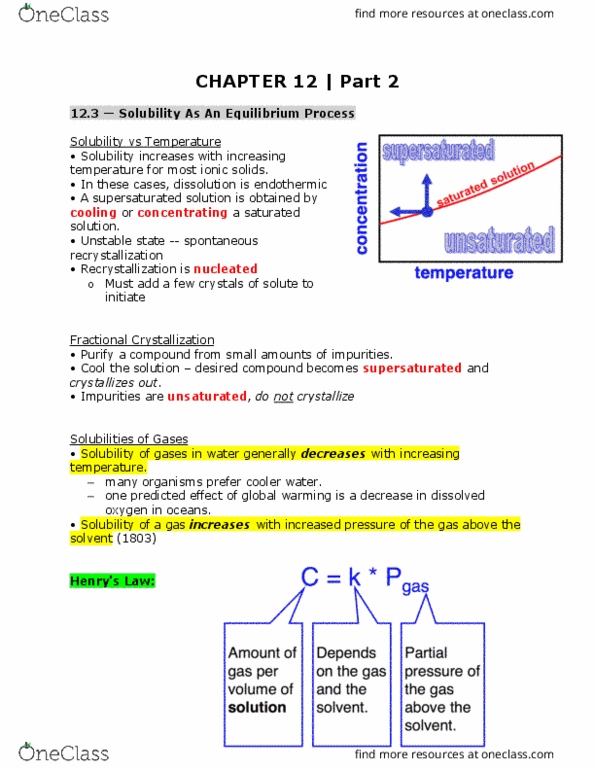
12. 1 types of solutions: intermolecular forces and solubility. A solution = a homogeneous mixture of two or more substances. Solution concentration: different measures of how much solute is dissolved in how much solvent. Small amount of solute: dilute solution large amount of solute: concentrated solution: the solubility (s) of a solute is the maximum amount that dissolves in a fixed quantity of solvent at a given temperature. Nacl solubility in water = 39. 12 g/100 ml (at 100 c) Agcl solubility in water = 0. 0021 g/100 ml (at 100 c) Intermolecular forces and the solution process: pure a has only a-a interactions, pure b has only b-b interactions, solution of a and b has a-a, b-b and a-b interactions. Dual polarity and effects on solubitility . Formation of ionic solutions: ionic solids form hydrated ions in solution, cations (+ve) = preferentially interact with water oxygen atoms (negative partial charge, anions (-ve) = preferentially interact with water hydrogen atoms (positive partial charge)