CHEM 1E03 Lecture 4: Lecture 4
CHEM 1E03 verified notes
4/40View all
Document Summary
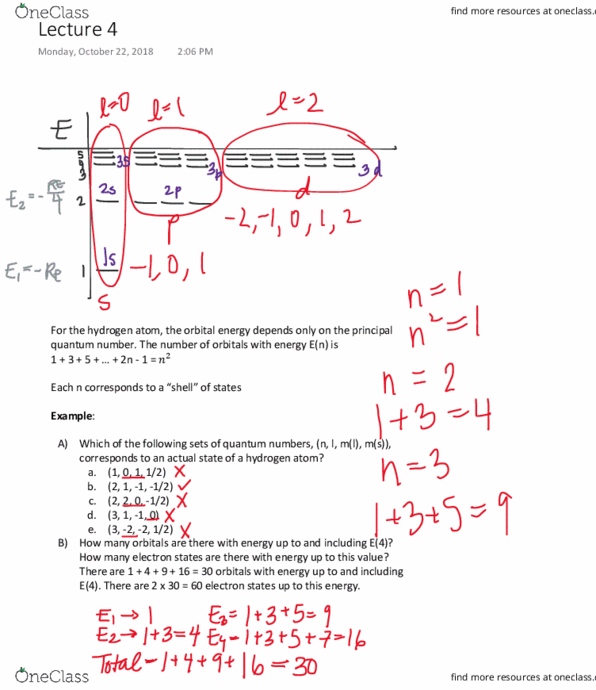
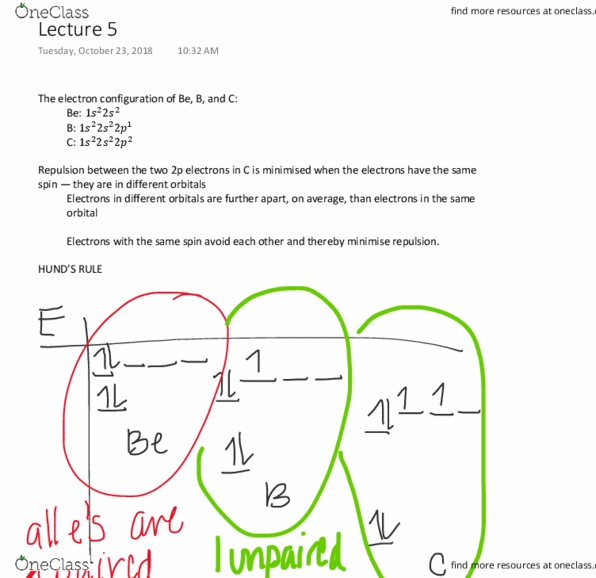
For the hydrogen atom, the orbital energy depends only on the principal quantum number. The number of orbitals with energy e(n) is. 1 + 3 + 5 + + 2n - 1 = # Each n corresponds to a shell of states. There are 1 + 4 + 9 + 16 = 30 orbitals with energy up to and including. There are 2 x 30 = 60 electron states up to this energy. An orbital of hydrogen corresponds to a wave function which (together with the spin) describes the state of the electron in the atom. A wave function is an oscillatory function of position about the nucleus. They can be positive or negative values or even complex. The square of the wave function at each position gives the likelihood of finding the electron in that location. Where the magnitude of the wave function is largest is where the electron is likely to be.