CHEM101 Lecture 7: January 21: Quantum Number review, d orbital shapes and energies, fourth quantum number, and multielecton atoms
CHEM101 verified notes
7/41View all
6
CHEM101 Lecture Notes - Lecture 6: Atomic Orbital, Azimuthal Quantum Number
7
CHEM101 Lecture 7: January 21: Quantum Number review, d orbital shapes and energies, fourth quantum number, and multielecton atoms
8

CHEM101 Lecture 8: January 23: effective charge, screening, orbitals, relative energy of orbitals, and electron configuration of the elements
Document Summary
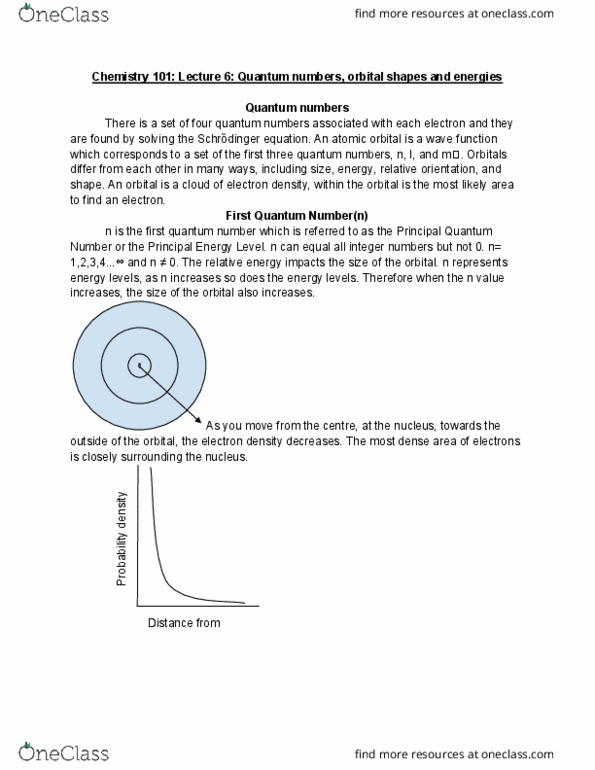
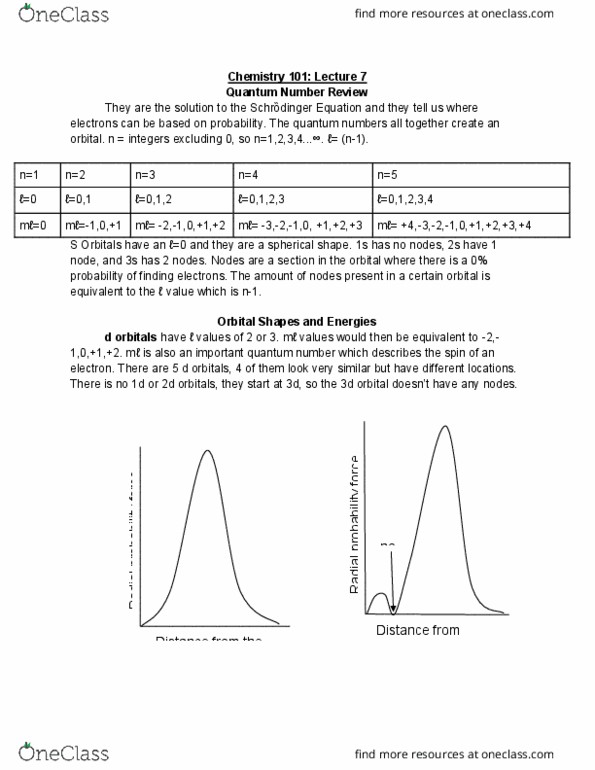
They are the solution to the schr dinger equation and they tell us where electrons can be based on probability. The quantum numbers all together create an orbital. n = integers excluding 0, so n=1,2,3,4 . =0,1,2,3,4 m =0 m =-1,0,+1 m = -2,-1,0,+1,+2 m = -3,-2,-1,0, +1,+2,+3 m = +4,-3,-2,-1,0,+1,+2,+3,+4. S orbitals have an =0 and they are a spherical shape. 1s has no nodes, 2s have 1 node, and 3s has 2 nodes. Nodes are a section in the orbital where there is a 0% probability of finding electrons. The amount of nodes present in a certain orbital is equivalent to the value which is n-1. Orbital shapes and energies d orbitals have values of 2 or 3. m values would then be equivalent to -2,- 1,0,+1,+2. m is also an important quantum number which describes the spin of an electron. There are 5 d orbitals, 4 of them look very similar but have different locations.


