CHM136H1 Lecture 12: syubstituted cycloalkanes
CHM136H1 verified notes
12/39View all
Document Summary
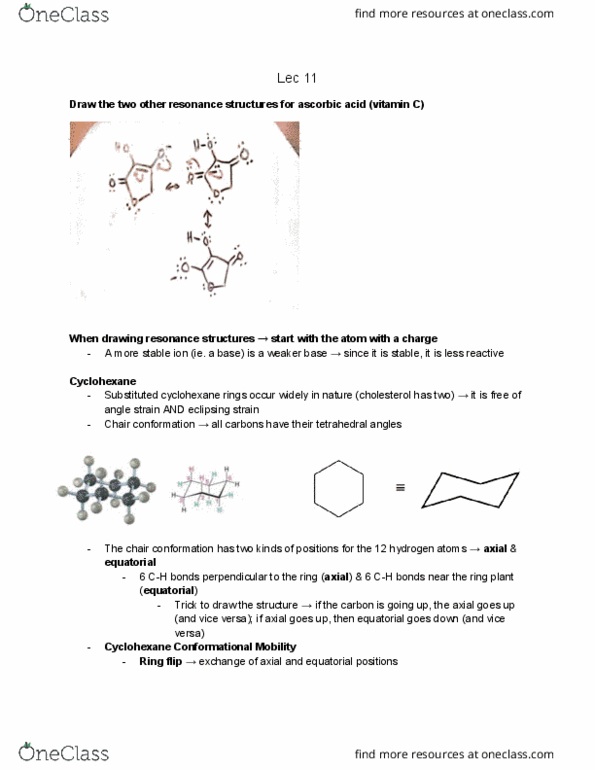
Cyclohexane ring rapidly flips between chair conformations at room temperature for monosubstituted cyclohexanes, the two conformers are no longer equally stable. 1-3-diaxial interactions steric strain in the axial conformation (because the methyl group is too close to the axial hydrogens) We must now take into consideration the effects of both substituents in both conformations. Both conformations have the exact same energy so the molecule exists as both conformers equally. The single gauche interaction causes the least amount of strain. Even more likely to be equatorial if the alkyl groups are even bigger (ie. instead of methyl, have ethyl, butyl etc. )