CHM136H1 Lecture 15: Chirality
CHM136H1 verified notes
15/39View all
Document Summary
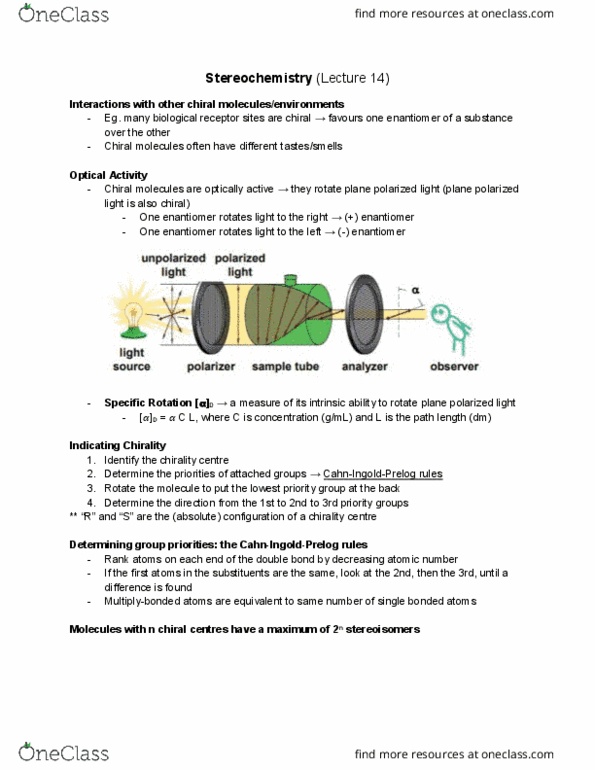
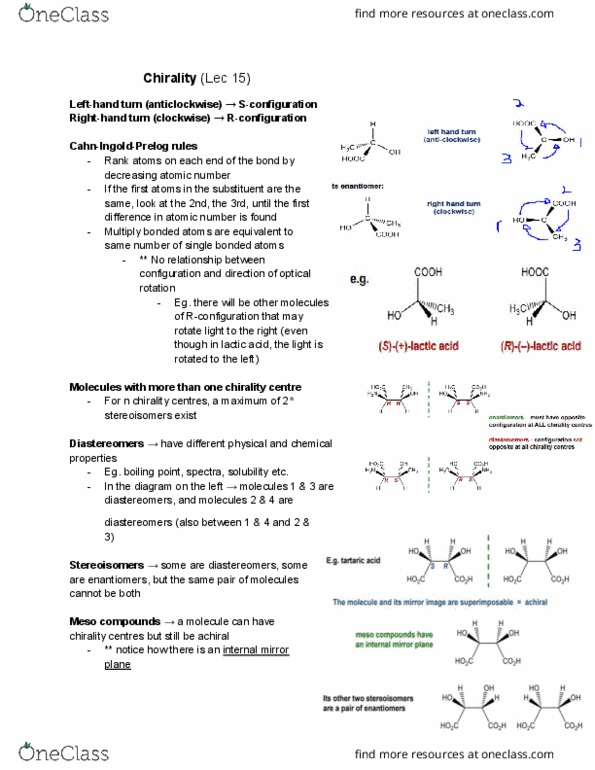
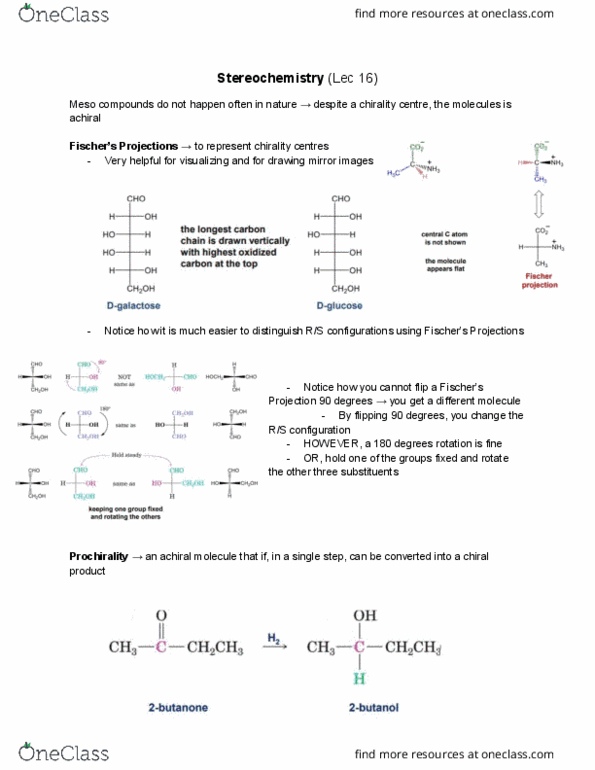
Rank atoms on each end of the bond by decreasing atomic number. If the first atoms in the substituent are the same, look at the 2nd, the 3rd, until the first difference in atomic number is found. Multiply bonded atoms are equivalent to same number of single bonded atoms. ** no relationship between configuration and direction of optical rotation. Eg. there will be other molecules of r-configuration that may rotate light to the right (even though in lactic acid, the light is rotated to the left) For n chirality centres, a maximum of 2n stereoisomers exist. Diastereomers have different physical and chemical properties. In the diagram on the left molecules 1 & 3 are diastereomers, and molecules 2 & 4 are diastereomers (also between 1 & 4 and 2 & Stereoisomers some are diastereomers, some are enantiomers, but the same pair of molecules cannot be both. Meso compounds a molecule can have chirality centres but still be achiral.