CHM136H1 Lecture Notes - Lecture 36: Lone Pair, Pyrrole, Aromaticity
86 views1 pages
Verified Note
30 Mar 2019
School
Department
Course
CHM136H1 verified notes
36/39View all
Document Summary
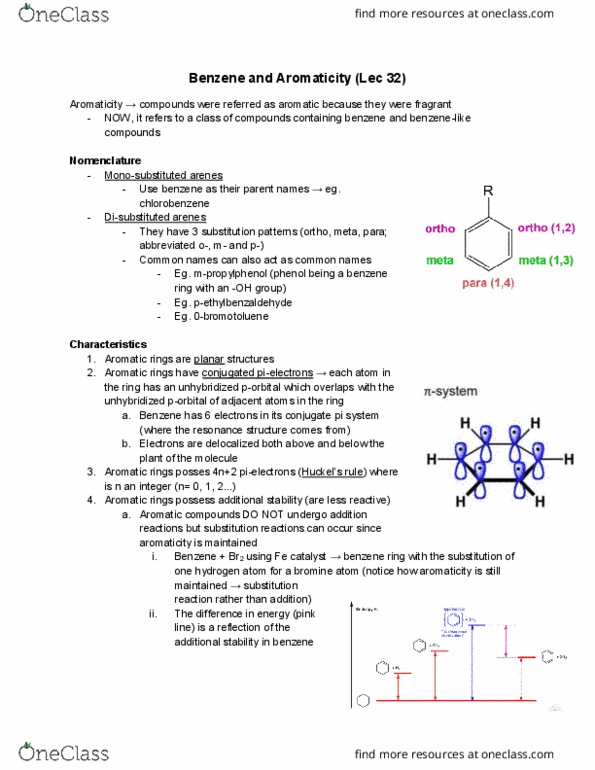
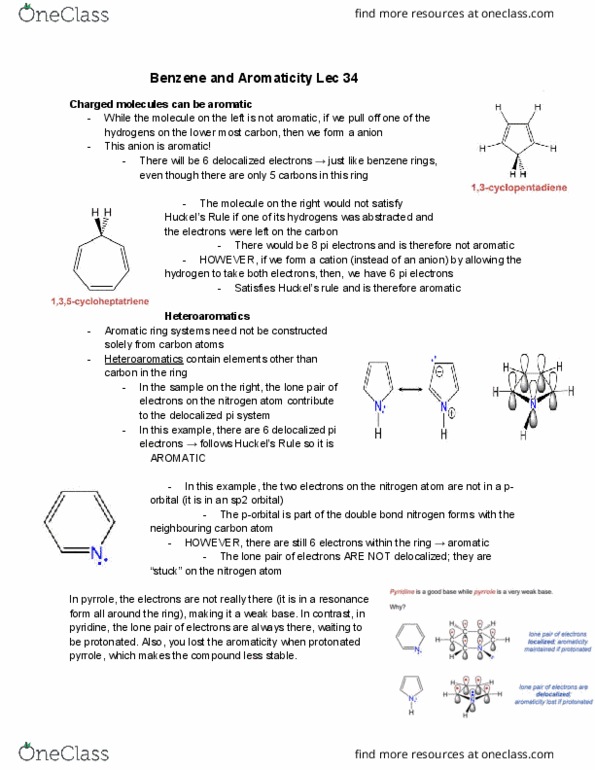
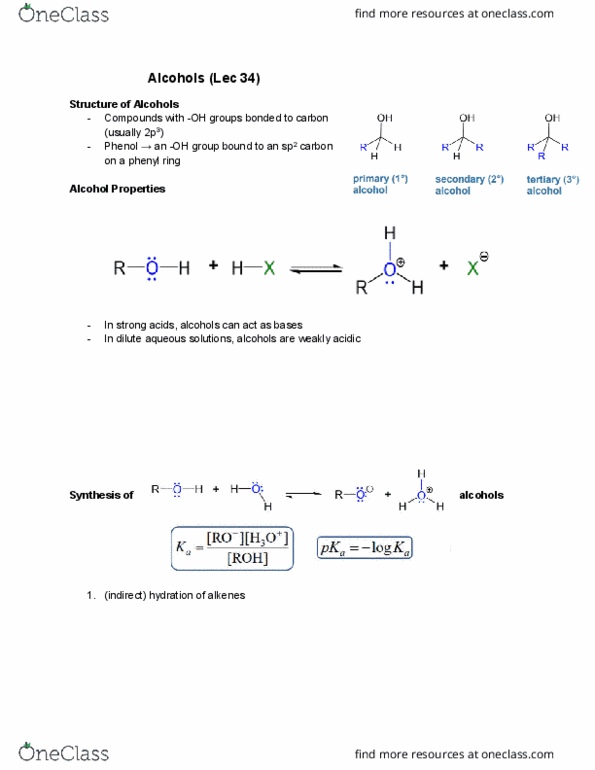
While the molecule on the left is not aromatic, if we pull off one of the hydrogens on the lower most carbon, then we form a anion. There will be 6 delocalized electrons just like benzene rings, even though there are only 5 carbons in this ring. The molecule on the right would not satisfy. Huckel"s rule if one of its hydrogens was abstracted and the electrons were left on the carbon. There would be 8 pi electrons and is therefore not aromatic. However, if we form a cation (instead of an anion) by allowing the hydrogen to take both electrons, then, we have 6 pi electrons. Satisfies huckel"s rule and is therefore aromatic. Aromatic ring systems need not be constructed solely from carbon atoms. Heteroaromatics contain elements other than carbon in the ring. In the sample on the right, the lone pair of electrons on the nitrogen atom contribute to the delocalized pi system.
Get access
Grade+20% off
$8 USD/m$10 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
40 Verified Answers
Class+
$8 USD/m
Billed $96 USD annually

Homework Help
Study Guides
Textbook Solutions
Class Notes
Textbook Notes
Booster Class
30 Verified Answers