CHM136H1 Lecture Notes - Lecture 35: Benzene, Chlorobenzene, Substitution Reaction
CHM136H1 verified notes
35/39View all
Document Summary
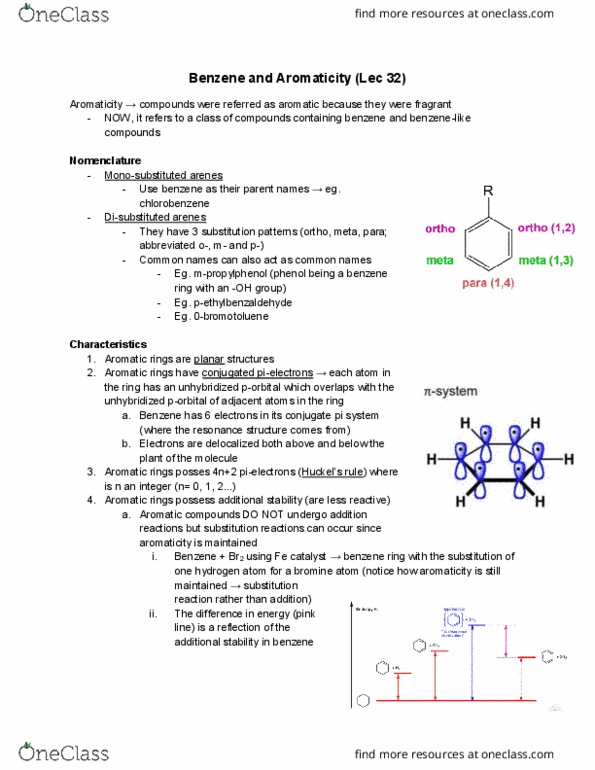
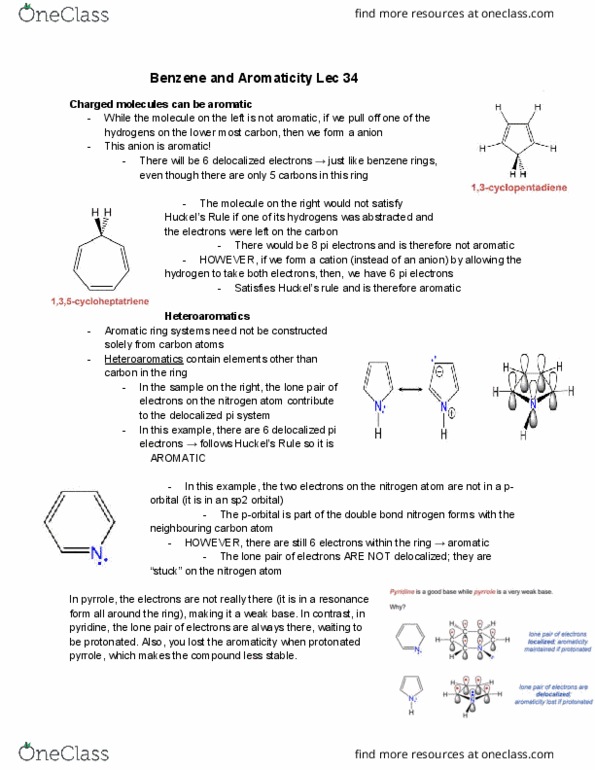
Aromaticity compounds were referred as aromatic because they were fragrant. Now, it refers to a class of compounds containing benzene and benzene-like compounds. Use benzene as their parent names eg. chlorobenzene. They have 3 substitution patterns (ortho, meta, para; abbreviated o-, m- and p-) Common names can also act as common names. Eg. m-propylphenol (phenol being a benzene ring with an -oh group) Benzene + br2 using fe catalyst benzene ring with the substitution of one hydrogen atom for a bromine atom (notice how aromaticity is still maintained substitution reaction rather than addition)